��Ŀ����
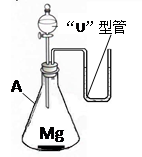
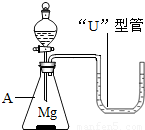
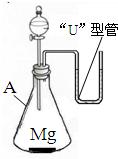 23������ͬѧ���øĽ���ϡ��Ũ�����ʵ��װ�������һ��Ȥζʵ�飮����һ��������þ������װ���У���A�м���������ϡ���ᣮA��������
23������ͬѧ���øĽ���ϡ��Ũ�����ʵ��װ�������һ��Ȥζʵ�飮����һ��������þ������װ���У���A�м���������ϡ���ᣮA����������ƿ
���ڷ�����ѧ��Ӧ�Ĺ����У����ܹ۲쵽��ʵ������������ɫ��þ�����٣������������ݣ�����
��������֪ʶ�������ڡ�U�����й۲쵽�������ԭ����þ��ϡ���ᷢ����ѧ��Ӧ�����������ų��������ȣ�ʹװ���ڵ�����ѹǿ�����������ѹ����ѹǿ��������£���U�����ڵ����Һ���½����Ҳ�Һ������
�����������Ը���þ��ϡ���ᷴӦ��ʵ�ʣ�þ��ϡ���ᷴӦ�����������ų�������ƿ����ѹ������������ѹ���ȷ�����з��������ǣ��Ӷ��ó���ȷ�Ľ��ۣ�
����⣺������������Ϊ����ƿ þ��ϡ���ᷴӦ�����������ų������������в����������ԭ���ǣ���ΪMg+2HCl=MgCl2+H2��ʹ��ƿ���������࣬�ҷ�Ӧ����ʹ�����������ͣ�ƿ����ѹ������������ѹ����ѹǿ��������£���U�����ڵ����Һ���½����Ҳ�Һ��������
�ʴ�Ϊ����ƿ������ɫ��þ�����٣������������ݣ����ȡ�þ��ϡ���ᷢ����ѧ��Ӧ�����������ų��������ȣ�ʹװ���ڵ�����ѹǿ�����������ѹ����ѹǿ��������£���U�����ڵ����Һ���½����Ҳ�Һ��������
�ʴ�Ϊ����ƿ������ɫ��þ�����٣������������ݣ����ȡ�þ��ϡ���ᷢ����ѧ��Ӧ�����������ų��������ȣ�ʹװ���ڵ�����ѹǿ�����������ѹ����ѹǿ��������£���U�����ڵ����Һ���½����Ҳ�Һ��������
�����������Ĺؼ���Ҫ����þ��ϡ���ᷴӦ��ʵ�ʣ���ΪMg+2HCl=MgCl2+H2��ʹ��ƿ���������࣬�ҷ�Ӧ����ʹ�����������ͣ�ƿ����ѹ������������ѹ����ѹǿ��������£���U�����ڵ����Һ���½����Ҳ�Һ��������ֻ�������˷�Ӧ��ʵ�ʲ��ܶ�����������ȷ���жϣ�

��ϰ��ϵ�д�
 ��У����ϵ�д�
��У����ϵ�д�
�����Ŀ