��Ŀ����
��1�����������������Ϊx
2NH3+H2SO4== (NH4)2SO4
34 98
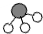
x 50g��9.8% 34/ x = 98/50g��9.8% (1��)
x =1.7g ����������Ϊ1.7g (1��)
��2��N% =�� 1.7g ��14/17��/100g��100% = 1.4% (2��)
��3��1.92%��4% (1��) ��1.4%﹤1.92% ���̷۲��ϸ� ��1�֣�
��4����������Ҫ����yg�����谷
100g��1.4% ﹢y��84/126 = (100g﹢y) ��1.92% (2��)
y = 0.8g ������Ҫ����0.8g�����谷 ��1�֣�
ij�������ú�Fe2O3������ʯұ��10t������2%��������������Ҫһ����̼�������Ƕ��٣���״���£�һ����̼������Ƕ��٣�����״���£�һ����̼���ܶ�Ϊ1.25kg/m3��

��ϰ��ϵ�д�
 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
�����Ŀ
 ��
��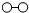 ��
�� �ֱ��ʾN2��H2��NH3���ڴ������棨��ͼ��
�ֱ��ʾN2��H2��NH3���ڴ������棨��ͼ�� ��ʾ�������棩N2��H2��Ӧ�ϳɰ��ķ�Ӧ���̿�������ͼ��ʾ���£�
��ʾ�������棩N2��H2��Ӧ�ϳɰ��ķ�Ӧ���̿�������ͼ��ʾ���£�

 2H2��+O2��
2H2��+O2��
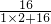 ��100%=88.89%
��100%=88.89%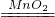 2H2O+O2�����ܲ������ٿ���������һ�⣬����ΪҲ�����������ַ�����������Կ���������õĽⷨ����д������
2H2O+O2�����ܲ������ٿ���������һ�⣬����ΪҲ�����������ַ�����������Կ���������õĽⷨ����д������