��Ŀ����
����Ŀ��ij�о���Ա����ͼ��ʾ���̽��С�ȼú�����������о�����ʵ���У���һ���Էֱ�����Ӧװ���� ����ˮ������Ȼ������ͨ�뺬��CO2��SO2��ȼú������O2������壬��ӦҺ���ѭ�����������������ڷ�Ӧװ������ѭ������ķ�ӦҺ���Ϸ�Ӧ���������ѭ��������ӦҺ�����������Ʊ�FeSO4��7H2O���塣

��1������װ����ˮ�²��˹��ߵ�ԭ���� ����װ�����ܷ�Ӧ�Ļ�ѧ����ʽΪ2X+2H2O+O2=2H2SO4����X����Է�������Ϊ ��
��2����Ӧװ���з�����Ӧ�Ļ�ѧ����ʽΪ ��
��3���������ѭ���Ժ���ӦҺ��������ȴ�����ˡ�ϴ��������T���Ƶ�FeSO47H2O������ҽ���ϳ��������� ��ѡ����ĸ����
A����״���״� B�����Ͳ� C��ȣ�� D��ƶѪ֢
��4��������װ��������������ȫ������β��1��β��2��һ�������´��ϳɼ״�CH3OH��ͬʱ��H2O���ɣ��÷�Ӧ�Ļ�ѧ����ʽΪ ��
���𰸡���1���¶ȹ����������ܽ��С�����׳������ 64��2��Fe + H2SO4 === FeSO4 + H2��
��3��D (4) CO2+3H2 ���� CH3OH+H2O
��������
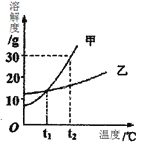
�������������װ����ˮ�²��˹��ߵ�ԭ�����¶ȹ��ߣ������ܽ��С�����׳����������װ�����ܷ�Ӧ�Ļ�ѧ����ʽΪ2X+2H2O+O2=2H2SO4����X����Է�������Ϊ=��98��2-2��18����2=64����Ӧװ���з�����Ӧ�Ļ�ѧ����ʽΪFe + H2SO4 === FeSO4 + H2�����������ѭ���Ժ���ӦҺ��������ȴ�����ˡ�ϴ��������T���Ƶ�FeSO47H2O������ҽ���ϳ���������ƶѪ֢����Ϊ�ܹ�����������ȱ������Ԫ�أ�������װ��������������ȫ������β��1��β��2��һ�������´��ϳɼ״�CH3OH��ͬʱ��H2O���ɣ��÷�Ӧ�Ļ�ѧ����ʽΪCO2+3H2 ���� CH3OH+H2O��