��Ŀ����
��2013?�γ�ģ�⣩��ͨ��ͭ��ͭ��п��ɣ��㷺���������ġ��ܲĵȣ�Ҳ���������е����ȣ�Ϊ�˲ⶨij��ͭ��Ʒ��ͭ�ĺ��������������ʵ�鷽����ȡ50.00g��ͭ��Ʒ������һ��δ֪Ũ�ȵ�ϡ���ᣮ�й�ʵ����������ͼ��עͼ1�е�������Ϊ����������/g��ͼ2��������Ϊ��Ӧ����Һ������/g�������궼Ϊ��������/g��
��1����Ʒ��ȫ��Ӧ����H2������
��2��ͼ��m��n�Ĺ�ϵ��m
��3����ͭ��Ʒ��ͭ�����������Ƕ��٣�д��������̣�����С�����һλ����
��4��ijͬѧ����ͼ�����ݼ��������������������Ϊ��
��100%=
��100%=10.03%�������ͬѧ�ļ������Ƿ���ȷ���粻��ȷָ�������ԭ��

��1����Ʒ��ȫ��Ӧ����H2������
0.5
0.5
����2��ͼ��m��n�Ĺ�ϵ��m
=
=
n�������������������������3����ͭ��Ʒ��ͭ�����������Ƕ��٣�д��������̣�����С�����һλ����
��4��ijͬѧ����ͼ�����ݼ��������������������Ϊ��
| m(HCl) |
| m(HCl��Һ) |
| m(HCl) |
| 198.25-m(zn) |
�ڼ������������ʱֻ���ǵ���Ӧ����Һ��ȥпԪ����������û�м�����������Ԫ�ص�����
�ڼ������������ʱֻ���ǵ���Ӧ����Һ��ȥпԪ����������û�м�����������Ԫ�ص�����
����������1����ͼ1��֪��Ʒ�������������������0.5g��
��2����ͭ��ֻ��п�ܹ������ᷴӦ�������������Կ����жϳ�m��n�Ĵ�С��ϵ��
��3��������������������Ϸ���ʽ�ɼ����п���������������������ͭ������������
��4�����ڷ�Ӧ�������������������е���Ԫ�������������У������ڼ����ϡ���������ʱӦ�ÿ��ǵ�п�����������ɵ����������������Ծݴ˽��
��2����ͭ��ֻ��п�ܹ������ᷴӦ�������������Կ����жϳ�m��n�Ĵ�С��ϵ��
��3��������������������Ϸ���ʽ�ɼ����п���������������������ͭ������������
��4�����ڷ�Ӧ�������������������е���Ԫ�������������У������ڼ����ϡ���������ʱӦ�ÿ��ǵ�п�����������ɵ����������������Ծݴ˽��
����⣺��1������ͼ1�е���Ϣ����֪����������������Ϊ0.5g��
��2����ͭ��ֻ��п�ܹ������ᷴӦ����������m��n ������Ʒ������пǡ�÷�Ӧʱ���ĵ����������������m��ֵ��ͼ2��n��ֵ��ȣ�
��3�����ͭ��Ʒ��п������Ϊx
Zn+2HCl�TZnCl2+H2��
65 2
x 0.5g
=
x=16.25g
��ͭ��Ʒ��ͭ�����������ǣ�
��100%=67.5%��
�𣺻�ͭ��Ʒ��ͭ������������67.5%��
��4����ͬѧ���������������������Ϊ��
��100%=
��100%=10.03%�����ڷ�Ӧ�����������������ڼ����ϡ���������ʱӦ�ÿ��ǵ�п�����������ɵ����������������������غ㶨�ɣ�ǡ����ȫ��Ӧʱ����ϡ���������=��Ӧ��������Һ������+�ų�����������-�μӷ�Ӧ����п��������
�ʴ�Ϊ����1��0.5��
��2������
��3��67.5%��
��4���ڼ������������ʱֻ���ǵ���Ӧ����Һ��ȥпԪ����������û�м�����������Ԫ�ص�������
��2����ͭ��ֻ��п�ܹ������ᷴӦ����������m��n ������Ʒ������пǡ�÷�Ӧʱ���ĵ����������������m��ֵ��ͼ2��n��ֵ��ȣ�
��3�����ͭ��Ʒ��п������Ϊx
Zn+2HCl�TZnCl2+H2��
65 2
x 0.5g
| 65 |
| x |
| 2 |
| 0.5g |
x=16.25g
��ͭ��Ʒ��ͭ�����������ǣ�
| 50.00g-16.25g |
| 50.00g |
�𣺻�ͭ��Ʒ��ͭ������������67.5%��
��4����ͬѧ���������������������Ϊ��
| m(HCl) |
| m(HCl��Һ) |
| m(HCl) |
| 198.25-m(zn) |
�ʴ�Ϊ����1��0.5��
��2������
��3��67.5%��
��4���ڼ������������ʱֻ���ǵ���Ӧ����Һ��ȥпԪ����������û�м�����������Ԫ�ص�������
���������������Ŀʱ�����ȣ�Ҫ��Ǻ��������ʵ��������������йؼ��㷽�����йػ�ѧ����ʽ�ļ��㷽�����Լ������غ㶨�ɽ�����֪ʶ��Ȼ��������������龰�����ѧ�����֪ʶ�ͼ��ܣ�ϸ�ĵؽ���̽�����������������Ŀ��Ҫ������ؽ��н��

��ϰ��ϵ�д�
�����Ŀ
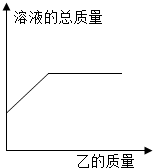 ��2013?�γ�ģ�⣩��һ�������ļ���Һ����������������ʵ���������Һ��������������ҵ�������ϵ��������ͼ���߱�ʾ���� ��������
��2013?�γ�ģ�⣩��һ�������ļ���Һ����������������ʵ���������Һ��������������ҵ�������ϵ��������ͼ���߱�ʾ���� �������� ��2013?�γ�ģ�⣩ʵ���ҳ��ñ��������������Ȼ����Һ��Ӧ��ȡ�����ĵ�������Ӧ�Ļ�ѧ����ʽΪ��NaNO2+NH4Cl=NaCl+N2+2H2O���˷�Ӧ�Ƿ��ȷ�Ӧ����ʵ��װ����ͼ��ʾ���Իش�
��2013?�γ�ģ�⣩ʵ���ҳ��ñ��������������Ȼ����Һ��Ӧ��ȡ�����ĵ�������Ӧ�Ļ�ѧ����ʽΪ��NaNO2+NH4Cl=NaCl+N2+2H2O���˷�Ӧ�Ƿ��ȷ�Ӧ����ʵ��װ����ͼ��ʾ���Իش�