��Ŀ����
 ����۳�Ϊ������ϲ����Ȼ��һЩ���������ñ��Ƿ�ð����������ۣ�һ�о���ѧϰС��Ϊ�˼���ij����۵���٣�����������̽����
����۳�Ϊ������ϲ����Ȼ��һЩ���������ñ��Ƿ�ð����������ۣ�һ�о���ѧϰС��Ϊ�˼���ij����۵���٣�����������̽����
[��������]
�������к�̼���80-93%��������4-14%��ˮ��2-4%����Ԫ�صȣ�
�ڵ�������Ũ���ᷴӦ���ɫ��
�۱��Ƿ���Ҫ�ɷ���̼��ƣ���������98%���ϣ���
[ʵ��]
ʵ��һ����ʢ�������������Ʒ���Թ��У��μ�����________����һ�ֻ�ѧҩƷ���ƣ����۲������Դ˼�����������٣�
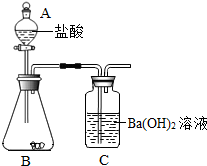
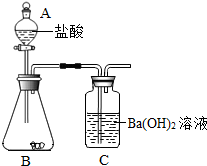
ʵ�������ͼ��ʾװ�òⶨ�������Ʒ��̼��Ƶĺ�����
ʵ�������
�ٰ�ͼ��������װ�ã��ý�Ƥ������B��Cʱ����Ҫ��________��Ȼ�����������Ѳ����ܲ��뽺Ƥ�ܣ����Ӻú�����������ԣ�
��ȷ��ȡa g�������Ʒװ������B�У���A��װ����������Ϊb%������m mL��
����B�ڵ��������Ʒ����μ������������
����ȫ��Ӧ��A��ʣ������n mL��C�е���Һ��________��ϴ�ӡ�����Ƶð�ɫ���������Ϊw g��
�ݼ�����Ʒ��̼��Ƶĺ�����
[����]
װ��B�з�����Ӧ�Ļ�ѧ����ʽΪ________�����ó���©������A����Һ©����ʢ�����ᣬ�ɷ�ֹ��װ��©������һ������________���ܹ�˵����Ʒ����ȫ��Ӧ������������________��������Ʒ��̼��Ƶ���������ʱ����Ҫ�õ���ʵ��������________��
[��˼]
�������Ʒ��̼��Ƶ�����������ʵ��ֵС������ܵ�ԭ����________������ţ���
a��ԭ���п��ܺ���̼��þ������
b��װ��B��CO2δ��ȫ����װ��C
c��װ��B���в���HCl����װ��C��
d��װ��C�в������������ͨ��δ�ܷ�
[��չ����]
��ͬѧ�����ʵ����в���Ҫװ��C�����õ�����ƽ����װ��A��B��Ӧǰ����������Ϳ��Եõ��������Ʒ��̼��Ƶĺ���������Ϊ����������________��
Ũ���� �Ѳ����ܿ���ˮ��ʪ ���� CaCO3+2HCl=CaCl2+CO2��+H2O ��ֹ���ɵ�������� װ��B��û�����ݲ��� aw bc ���������غ㶨�ɣ�װ��A��C��Ӧǰ������������CO2������
��������������ṩ����Ϣ���з������������������ϻ��ƣ���������ʱ���Խ����ܿ���ˮ��ʪ����ȥ�����Թ������ʹ�ù��˵ķ�����̼����������ᷴӦ�����Ȼ��ơ�ˮ�Ͷ�����̼��ʹ�÷�Һ©���ܿ��Ʒ�Ӧ�Ľ��У�Ҫ����̼��Ƶ�������������Ҫ֪������۵�����������̼��Ƶ����������ݾ���������������ƫ�͵�ԭ����з������ݴ˽����У�
���ʵ��һ�����ڵ����ʺ�Ũ���ᷴӦ���ƣ��ʿ���ʹ��Ũ�����������۵���٣����Ũ���
ʵ���������Ҫ�Ѳ����ܺͽ�Ƥ����������������ʹ��ˮ�������ܿ���ʪ������Ѳ����ܿ���ˮ��ʪ��
������������ᷴӦ�����Һ�к��в����Թ��壬����ʹ�ù��˵ķ�����ȥ��������ˣ�
������̼����������ᷴӦ�����Ȼ��ơ�ˮ�Ͷ�����̼��ʹ�÷�Һ©�����Կ���Һ������٣��Ӷ����Ʒ�Ӧ�Ľ��У���ȫ��Ӧ��װ��B��û�����ݲ�����Ҫ������Ʒ��̼��Ƶ���������ʱ����Ҫ֪����Ʒ����������Ҫ���̼��Ƶ�������������w�������̼��Ƶ����������CaCO3+2HCl=CaCl2+CO2��+H2O����ֹ���ɵ�������죻װ��B��û�����ݲ�����a��w��
��˼��a��ԭ���п��ܺ���̼��þ�����ʣ�̼��þ�������ᷴӦ���ɶ�����̼���Ӷ����̼��Ƶĺ�������a����
b��װ��B��CO2δ��ȫ����װ��C���ᵼ�¼����̼��Ƶ�����ƫС������̼��Ƶĺ���ƫ�ͣ���b��ȷ��
c��װ��B���в���HCl����װ��C�У��ᵼ�����ɵ�̼�ᱵ������ƫС���Ӷ�����Ķ�����̼������ƫС������̼��Ƶ�����ƫС����c��ȷ��
d��װ��C�в������������ͨ��δ�ܷ⣬���¿����еĶ�����̼����C�У�����Ķ�����̼������ƫ�Ӷ�̼��Ƶ�����ƫ��d����
���bc��
��չ���죺�÷�Ӧ�����ɵ��Ƕ�����̼���������ݷ�Ӧǰ���������ȷ��������̼���������Ӷ����м��㣬������������غ㶨�ɣ�װ��A��C��Ӧǰ������������CO2��������
���������⿼���˲�������ij���ʵĺ�����ʵ����ƣ���ɴ��⣬������������ṩ����Ϣ������е�֪ʶ���У�
��������������ṩ����Ϣ���з������������������ϻ��ƣ���������ʱ���Խ����ܿ���ˮ��ʪ����ȥ�����Թ������ʹ�ù��˵ķ�����̼����������ᷴӦ�����Ȼ��ơ�ˮ�Ͷ�����̼��ʹ�÷�Һ©���ܿ��Ʒ�Ӧ�Ľ��У�Ҫ����̼��Ƶ�������������Ҫ֪������۵�����������̼��Ƶ����������ݾ���������������ƫ�͵�ԭ����з������ݴ˽����У�
���ʵ��һ�����ڵ����ʺ�Ũ���ᷴӦ���ƣ��ʿ���ʹ��Ũ�����������۵���٣����Ũ���
ʵ���������Ҫ�Ѳ����ܺͽ�Ƥ����������������ʹ��ˮ�������ܿ���ʪ������Ѳ����ܿ���ˮ��ʪ��
������������ᷴӦ�����Һ�к��в����Թ��壬����ʹ�ù��˵ķ�����ȥ��������ˣ�
������̼����������ᷴӦ�����Ȼ��ơ�ˮ�Ͷ�����̼��ʹ�÷�Һ©�����Կ���Һ������٣��Ӷ����Ʒ�Ӧ�Ľ��У���ȫ��Ӧ��װ��B��û�����ݲ�����Ҫ������Ʒ��̼��Ƶ���������ʱ����Ҫ֪����Ʒ����������Ҫ���̼��Ƶ�������������w�������̼��Ƶ����������CaCO3+2HCl=CaCl2+CO2��+H2O����ֹ���ɵ�������죻װ��B��û�����ݲ�����a��w��
��˼��a��ԭ���п��ܺ���̼��þ�����ʣ�̼��þ�������ᷴӦ���ɶ�����̼���Ӷ����̼��Ƶĺ�������a����
b��װ��B��CO2δ��ȫ����װ��C���ᵼ�¼����̼��Ƶ�����ƫС������̼��Ƶĺ���ƫ�ͣ���b��ȷ��
c��װ��B���в���HCl����װ��C�У��ᵼ�����ɵ�̼�ᱵ������ƫС���Ӷ�����Ķ�����̼������ƫС������̼��Ƶ�����ƫС����c��ȷ��
d��װ��C�в������������ͨ��δ�ܷ⣬���¿����еĶ�����̼����C�У�����Ķ�����̼������ƫ�Ӷ�̼��Ƶ�����ƫ��d����
���bc��
��չ���죺�÷�Ӧ�����ɵ��Ƕ�����̼���������ݷ�Ӧǰ���������ȷ��������̼���������Ӷ����м��㣬������������غ㶨�ɣ�װ��A��C��Ӧǰ������������CO2��������
���������⿼���˲�������ij���ʵĺ�����ʵ����ƣ���ɴ��⣬������������ṩ����Ϣ������е�֪ʶ���У�

��ϰ��ϵ�д�
 �»����ܶ�Ա��ϵ�д�
�»����ܶ�Ա��ϵ�д� ����ͼ����ּ��������ҵ֣�ݴ�ѧ������ϵ�д�
����ͼ����ּ��������ҵ֣�ݴ�ѧ������ϵ�д�
�����Ŀ
 ��2013?��ͨһģ������۳�Ϊ������ϲ����Ȼ��һЩ���������ñ��Ƿ�ð����������ۣ�һ�о���ѧϰС��Ϊ�˼���ij����۵���٣�����������̽����
��2013?��ͨһģ������۳�Ϊ������ϲ����Ȼ��һЩ���������ñ��Ƿ�ð����������ۣ�һ�о���ѧϰС��Ϊ�˼���ij����۵���٣�����������̽����