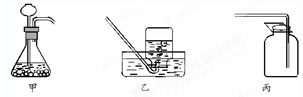
��Ŀ����
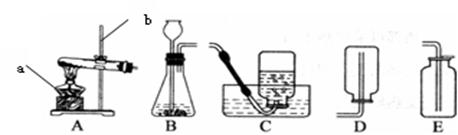
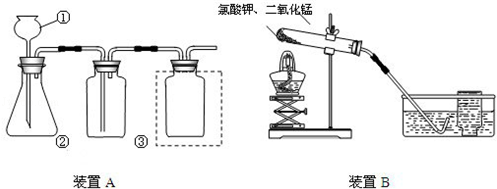
ʵ����������װ�ã������Ҫ��ش����⣺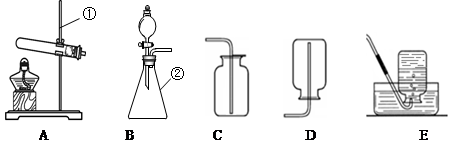
��1��д���б�����������ƣ��� ���� ��
��2�����Bװ�õ������Եķ����ǣ���ס�Ҳർ���ܣ� �����Һ©���м�������ˮ�����۲쵽��Һ©��ĩ�˳����ȶ���ˮ������װ�ò�©����
��3��ʵ�����ù���������Һ�Ͷ���������ȡ�����Ļ�ѧ����ʽΪ �� ѡ�õķ���װ��Ϊ (�����)��
��4��ʵ�����ø��������ȡ������������֤����ȼ�ղ����ʵ�飬ѡ�õ��ռ�װ��Ϊ (�����)������жϸ�װ�����ռ������� ��
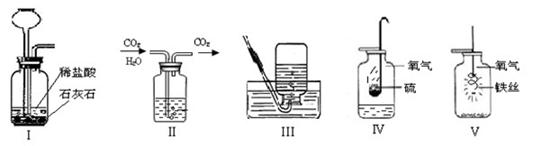
��5��ʵ�����ø��������ȡ����������ˮ���ռ�����Ҫʵ�鲽���У���װҩƷ���Ƽ��װ�������ԣ��Ǽ��ȣ���װ�ù̶�������̨�ϣ���Ϩ��ƾ��ƣ����ռ����壻�˽������Ƴ�ˮ�ۡ���ȷ�IJ���˳��Ϊ
| A���ŢƢǢȢɢʢ� | B���ƢŢȢǢʢɢ� |
| C���ŢƢȢǢʢɢ� | D���ƢŢȢǢʢˢ� |
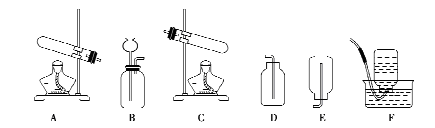
��1��������̨������ƿ��
��2����Һ©��������
��3��2H2O2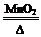 2H2O+O2�� B
2H2O+O2�� B
��4��C�� �������ǵ�ľ�����뼯��ƿ��ƿ�ڣ���ľ����ȼ�������ռ�����
��5��D
�������������
��2��������������ã������е����岻����٣�ˮҲ������룬��ĩ�˳����ȶ���ˮ����˵����©����
��3�����巢��װ�÷����֣��̹̼����ͣ�A����Һ�������ͣ�B������������ΪҺ�塢��������Ϊ���壬�ҷ�Ӧ������ȣ����Է���װ��ѡB��
��4������ȼ�ղ���Ϊ������̼��ˮ��Ϊ����֤��ˮ���ɣ�����ƿ�е����������Ǹ���������ˮ�ļ��飬����ֻ���������ſ������ռ����塣���������ǽ������ǵ�ľ�����뼯��ƿ��ƿ�ڣ���ľ����ȼ�������ռ�����
��5��ʵ������ȡ��������Ҫ��������ɼ���Ϊ����װ��������Ϩ��г��Ϊ����ׯ��������Ϣ�����ݴˣ�ѡD����ע����ǣ�����������ܵ������������������ˮ���е�ˮ���������Թܣ������Թ�ը�ѡ�
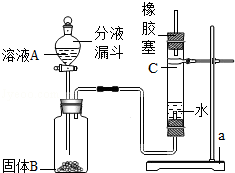
���㣺ʵ������ȡ������װ��ѡ����������������

 �߽�������ϵ�д�
�߽�������ϵ�д�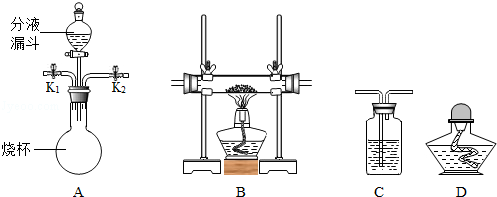
 4Cu+CO2+2H2O
4Cu+CO2+2H2O