��Ŀ����
��2012?��������ģ����ش������й�����֪ʶ�����⣺
��1��ʵ������Ũ���ᣨ������������Ϊ98%���ܶ�Ϊ1.84g/cm3������300g������������11%��ϡ���ᣮ���������һλС����
a���㣺��Ũ����
b��ȡ���ù��Ϊ50ml����Ͳ��ȡŨ���ᣮ
cϡ�ͣ�ϡ��Ũ����ʱ��һ��Ҫ��Ũ��������ע��ˮ�в����Ͻ��裮
��2��Fe��Cu��HCl��Ca��OH��2��Fe2O3��CO2�������в�����H2SO4������ѧ��Ӧ����
��3��ijװŨ��������䳵���ʻ���������Ũ��������й©�¹ʣ�Ϊʹ�¹ʶԻ�����ɵ�Ӱ�쾡���ܽ��ͣ����д�ʩ����Ѵ�ʩΪ
A���ô���ˮ��ϴ��B������ʯ�ҡ�C����ɳ����D�����ռ
��1��ʵ������Ũ���ᣨ������������Ϊ98%���ܶ�Ϊ1.84g/cm3������300g������������11%��ϡ���ᣮ���������һλС����
a���㣺��Ũ����
18.3
18.3
ml��ˮ266.3
266.3
ml��b��ȡ���ù��Ϊ50ml����Ͳ��ȡŨ���ᣮ
cϡ�ͣ�ϡ��Ũ����ʱ��һ��Ҫ��Ũ��������ע��ˮ�в����Ͻ��裮
��2��Fe��Cu��HCl��Ca��OH��2��Fe2O3��CO2�������в�����H2SO4������ѧ��Ӧ����
Cu��HCl��CO2
Cu��HCl��CO2
���ѧʽ����д������һ���������ֽⷴӦ�Ļ�ѧ����ʽ3H2SO4+Fe2O3=Fe2��SO4��3+3H2O
3H2SO4+Fe2O3=Fe2��SO4��3+3H2O
����3��ijװŨ��������䳵���ʻ���������Ũ��������й©�¹ʣ�Ϊʹ�¹ʶԻ�����ɵ�Ӱ�쾡���ܽ��ͣ����д�ʩ����Ѵ�ʩΪ
B
B
��A���ô���ˮ��ϴ��B������ʯ�ҡ�C����ɳ����D�����ռ
��������1����Һϡ��ǰ��������������䣬�ݴ˿�����������Ũ�������������Һ������=Ũ���������+ˮ��������Ȼ����
=�ܶȿ�����������Ũ���ᡢˮ�������
��2���������ʼ�ķ�Ӧ�����жϣ�
��3�����ݸ���ʩѡ�õ����ʵ����ʼ���ɵĺ���жϣ�
| ���� |
| ��� |
��2���������ʼ�ķ�Ӧ�����жϣ�
��3�����ݸ���ʩѡ�õ����ʵ����ʼ���ɵĺ���жϣ�
����⣺��1������Ҫ98%�����������Ϊx��
����ϡ��ǰ�����ʵ���������ɵã�300g��11%=x��98%
��ã�x��33.7g��
��Ҫˮ������Ϊ��ϡ�ͺ���Һ����-Ũ���������=300g-33.67g=266.3g��
����V=
�ɵã�
��ҪŨ��������Ϊ��
��18.3mL��
��Ҫˮ�����Ϊ��
=266.3mL��
���18.3��266.3��
��2����Fe��Cu��HCl��Ca��OH��2��Fe2O3��CO2�������У����ܺ�ϡ���ᷢ���û���Ӧ����������ͭ��ϡ�����Ӧ��HCl�����ᣬ����ϡ���ᷴӦ�����������Ǽ�ܺ�ϡ���ᷢ���кͷ�Ӧ���������ܺ�ϡ���ᷢ�����ֽⷴӦ������������ˮ��CO2���������壬���������ᷴӦ��������̼���Σ��ܺ�ϡ���ᷴӦ���ɶ�����̼���ʲ�����
H2SO4������ѧ��Ӧ����Cu��HCl��CO2��
���Cu��HCl��CO2��3H2SO4+Fe2O3=Fe2��SO4��3+3H2O��
��3��Ũ��������й©��ˮ��ϴ����ɳ�����Ƕ��������Ũ�������������Ⱦ���ռ����ǿ��ʴ�ԣ�����ɶ�����Ⱦ����ѡ�ó������Ե���ʯ��������Ũ���ᣮ
�ʴ�Ϊ��B��
����ϡ��ǰ�����ʵ���������ɵã�300g��11%=x��98%
��ã�x��33.7g��
��Ҫˮ������Ϊ��ϡ�ͺ���Һ����-Ũ���������=300g-33.67g=266.3g��
����V=
| m |
| �� |
��ҪŨ��������Ϊ��
| 33.67g |
| 1.84g/cm3 |
��Ҫˮ�����Ϊ��
| 266.3g |
| 1.0g/cm3 |
���18.3��266.3��
��2����Fe��Cu��HCl��Ca��OH��2��Fe2O3��CO2�������У����ܺ�ϡ���ᷢ���û���Ӧ����������ͭ��ϡ�����Ӧ��HCl�����ᣬ����ϡ���ᷴӦ�����������Ǽ�ܺ�ϡ���ᷢ���кͷ�Ӧ���������ܺ�ϡ���ᷢ�����ֽⷴӦ������������ˮ��CO2���������壬���������ᷴӦ��������̼���Σ��ܺ�ϡ���ᷴӦ���ɶ�����̼���ʲ�����
H2SO4������ѧ��Ӧ����Cu��HCl��CO2��
���Cu��HCl��CO2��3H2SO4+Fe2O3=Fe2��SO4��3+3H2O��
��3��Ũ��������й©��ˮ��ϴ����ɳ�����Ƕ��������Ũ�������������Ⱦ���ռ����ǿ��ʴ�ԣ�����ɶ�����Ⱦ����ѡ�ó������Ե���ʯ��������Ũ���ᣮ
�ʴ�Ϊ��B��
������������Ҫ��������Һ�����ơ����ʵ����ʺͻ�ѧ����ʽ����д�ȷ����֪ʶ����д��ѧ����ʽʱҪע����ѭ�����غ㶨�ɣ�

��ϰ��ϵ�д�
 ͬ����չ�Ķ�ϵ�д�
ͬ����չ�Ķ�ϵ�д�
�����Ŀ
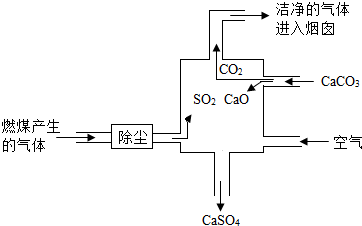 ��2012?��������ģ����ͼ��ijȼú���糧����������װ��ʾ��ͼ������˵������ȷ���ǣ�������
��2012?��������ģ����ͼ��ijȼú���糧����������װ��ʾ��ͼ������˵������ȷ���ǣ�������