��Ŀ����
��2004?���ݣ��ҹ�Ŀǰʹ�õ�ȼ����Ҫ��ú��ʯ�ͣ��������úȼ��ʱ���ɵ�SO2����Ⱦ��������1��ij�������糧ÿ��ȼ�պ���1.6%��ú100t����ú�е���ȫ��ת��ΪSO2����ó�ÿ�����SO2______t��Ϊ��ֹSO3��Ⱦ���ɽ�SO2ͨ��ʯ����������Ca��HSO3��2����÷�Ӧ�Ļ�ѧ����ʽΪ______��
��2�����ұ��涨��ҵ������SO2�������ó���0.15mg/m3����ҵ�ϲ���SO2�ĺ���ʱ�����Ը��ݷ�Ӧ��SO2+2H2O+I2=H2SO4+2HI����ȡ�ó�������Ʒ1000L����0.0254%�ĵ⣨12����Һ2g����ȫ��Ӧ���Լ���ó��ŷŵķ�����SO2�ĺ����Ƿ���Ϲ��ұ���
���𰸡����������ݷ�Ӧ��������P�������غ㶨�ɿ�����ȷ����д��ѧ����ʽ��������Ԫ�ص������Ͷ�����������Ԫ�ص��������������Լ���ó�������������������ݷ�Ӧ�Ļ�ѧ����ʽ��������ó��ŷŵķ�����SO2�ĺ����Ƿ���Ϲ��ұ���
����⣺��1��ÿ��ʹ��ú���е��������Ϊ100t×1.6%�����ڶ�����������Ԫ�ص���������Ϊ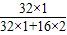 ×100%=50%�����Ըó�ÿ������������������Ϊ��100t×1.6%÷50%=3.2t�����3.2��
×100%=50%�����Ըó�ÿ������������������Ϊ��100t×1.6%÷50%=3.2t�����3.2��
����������������Ʒ�Ӧ��������������ƣ���Ӧ�Ļ�ѧ����ʽΪ��2SO2+Ca��OH��2�TCa��HSO3��2
��2����������������ΪX��
SO2+2H2O+I2=H2SO4+2HI
64 254
X 2g×0.0254%
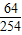 =
=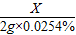
X=1.28×10-4g
��l000L������SO2������Ϊ1.28×10-4g��
1.28×10-4g=O.128mg/m3��0.15mg/m3�����Ϲ��ұ���
�����������Ҫ����������ط���ļ��㷽����ֻ����������ط���ļ��㷽������ͨ������ó���ȷ�Ĵ𰸣�
����⣺��1��ÿ��ʹ��ú���е��������Ϊ100t×1.6%�����ڶ�����������Ԫ�ص���������Ϊ
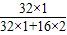 ×100%=50%�����Ըó�ÿ������������������Ϊ��100t×1.6%÷50%=3.2t�����3.2��
×100%=50%�����Ըó�ÿ������������������Ϊ��100t×1.6%÷50%=3.2t�����3.2������������������Ʒ�Ӧ��������������ƣ���Ӧ�Ļ�ѧ����ʽΪ��2SO2+Ca��OH��2�TCa��HSO3��2
��2����������������ΪX��
SO2+2H2O+I2=H2SO4+2HI
64 254
X 2g×0.0254%
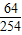 =
=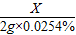
X=1.28×10-4g
��l000L������SO2������Ϊ1.28×10-4g��
1.28×10-4g=O.128mg/m3��0.15mg/m3�����Ϲ��ұ���
�����������Ҫ����������ط���ļ��㷽����ֻ����������ط���ļ��㷽������ͨ������ó���ȷ�Ĵ𰸣�

��ϰ��ϵ�д�
 Ӧ����㲦ϵ�д�
Ӧ����㲦ϵ�д� ״Ԫ����ϵ�д�
״Ԫ����ϵ�д� ͬ������ϵ�д�
ͬ������ϵ�д�
�����Ŀ