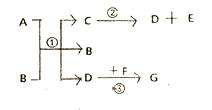
��Ŀ����
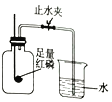
����Ŀ����ͼ��ʵ�����ú���ȼ�������Բⶨ����������������װ�á�
��1��д������ȼ�յķ��ű���ʽ��_______��
��2��ʵ��ԭ�������ں���ȼ�����Ŀ����е�������ʹƿ��_______��С���ձ��е�ˮ����������ƿ����װ�õ����������ã������淶������Ͳ��������ƿ��ˮ��������ܴ��Բ�ÿ����������ĺ�����
[�������]��ʵ�����ϱ�����ȼ�չ����е����������������7%ʱ������������ȼ�գ����ͨ������ʵ�飬�������������ֵ���ϴ�
[ʵ��Ľ�]
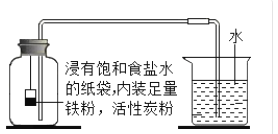
I���������ڿ����������ԭ���������ͼ��ʾʵ��װ�ã��ٴβⶨ�����������ĺ�����װ���б���ʳ��ˮ������̿����������⡣
�������ʵ���������±���
���� ��Ŀ | ʵ��ǰ | ʵ��� | |
�ձ���ˮ����� | �ձ���ʣ��ˮ����� | ����ƿ���۳�������͵��ܵ��ݻ� | |
���/mL | 80.0 | 54.5 | 126.0 |
[��������]
��1����������̷������ӵĻ�ѧ��Ӧ������������������ˮ��Ӧ����������������д���÷�Ӧ�����ֱ���ʽ��_________��
��2�����ݱ������ݼ��㣬�Ľ�ʵ����õĿ��������������������_____����������ȷ��0.1%����
��3����ʵ��ԭ���Ƕȷ������Ľ����ʵ������ǰ��ȷ�ȸ��ߵ�ԭ���ǣ�
��_________________��
��_________________��
���𰸡�![]() ��ѹ ����ˮ������������������ 20.2% ���Ļ�������ʹ����ƿ�е��������ĸ����ף�ʹʵ������ȷ ����ʱ���ǵ������ݻ��Ϳ۳��������ļ���ƿ�ݻ���ʹʵ������ȷ
��ѹ ����ˮ������������������ 20.2% ���Ļ�������ʹ����ƿ�е��������ĸ����ף�ʹʵ������ȷ ����ʱ���ǵ������ݻ��Ϳ۳��������ļ���ƿ�ݻ���ʹʵ������ȷ
��������
��1������ȼ���������������ף����ű���ʽΪ��![]() ��
��
��2������ȼ���������������ɵ������������ǹ��壬ʹƿ�ڵ���ѹ��С��ˮ������
[��������]
��1������������ˮ��Ӧ�����������������÷�Ӧ�����ֱ���ʽΪ������ˮ��������������������
��2�����ݷ�Ӧǰ���ձ���ˮ������仯���Կ��������ĵ�����������ǣ�80-54.5=25.5mL�����������������Ϊ��![]() ��
��
��3��
��ʹ�����Ļ�����������������ʹ�������ĵĸ�Ϊ���ף�ʵ������ȷ��
������ʱ���ǵ����ݻ��Ϳ۳��������ļ���ƿ�������ʹʵ������Ϊȷ��
��������Ļ�������ʹ����ƿ�е��������ĸ�Ϊ���ף�ʹʵ������ȷ��������ʱ���ǵ������ݻ��Ϳ۳��������ļ���ƿ�ݻ���ʹʵ������ȷ��

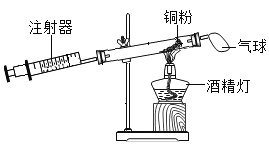
 ��У����ϵ�д�
��У����ϵ�д�����Ŀ��ij��ѧ��ȤС�鷢��ͭƬ�ڼ��ȵ������¿�����Ũ���ᷴӦ���������ݲ��ŵ��̼�����ζ��ͭƬ�ܽ⣬��Һ����ɫ���������ʵ��̽����
��������⣩������������ʲô��
���������ϣ�SO2��һ����ɫ���д̼�����ζ���ж�������
��������룩����һ��SO2���������SO2��H2����������SO2��CO2������Ϊ�����____�������ȷ����������������ͬʱ�������������ܵ�ԭ����_____��
��������⣩��ɫ��Һ��������ʲô��
��������룩����һ��CuSO4���������CuSO4��H2SO4��
������ʵ�飩ȡһ������Ӧ�����Һ����ϡ�ͣ�����װ��A��B��֧�Թ��У�Ȼ���������ʵ�飬������±���
���� | ʵ����� | ʵ������ | ʵ����� |
����һ | ���Թ�A�м���������CuO��ĩ | ______________ | ��Һ�к���H2SO4 |
����� | ���Թ�B�м��������NaOH��Һ | ______________ | ��Һ�к���CuSO4 |
��̽�����ۣ�ͨ��ʵ�飬�������ȷ��
��1��д������һ������Ӧ�Ļ�ѧ����ʽ��___________________________��
��2����ɲ���ƽ����ʽ��Cu+H2SO4��Ũ��________________________��
����˼���ۣ����ݻ�ѧ����ʽ��С����Ϊ��ҵ�Ͽ�����ͭ��Ũ���ᷴӦ������ȡ����ͭ��С����Ϊ�����ˣ�������_________________________��