��Ŀ����
ijˮ�೧�����ң�Ϊ�˲ⶨij��ɽʯ��ʯ��̼��Ƶ�����������ȡʯ��ʯ��Ʒ������ϡ�������ձ��з�Ӧ������ʯ��ʯ��Ʒ�����ʲ���ϡ���ᷴӦҲ������ˮ�����й�ʵ���������±���| ��Ӧǰ | ��Ӧ�� | ||
| ʵ �� �� �� | �ձ���ϡ���� ������ | ʯ��ʯ��Ʒ ������ | �ձ������л� ��������� |
| 150g | 12g | 157.6g | |
��2�����ʯ��ʯ��̼��Ƶ�����������
���𰸡���������1�����������غ㶨�ɿ�֪����Ӧ��ȷ�Ӧǰ���ٵ����������ɶ�����̼��������
��2�����ݻ�ѧ����ʽ�ɶ�����̼���������Լ����ʯ��ʯ��̼��Ƶ����������������ʯ��ʯ��̼��Ƶ�����������
����⣺��1�����������غ㶨�ɣ�������̼������Ϊ��150g+12g-157.6g=4.4g��
��2�����ʯ��ʯ��Ʒ��̼��Ƶ�����Ϊx��
CaCO3+2HCl�TCaCl2+H2O+CO2��
100 44
x 4.4g
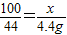 ��x=10g
��x=10g
��ʯ��ʯ��̼��Ƶ���������Ϊ��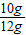 ×100%��83.3%
×100%��83.3%
�𣺸�ʯ��ʯ��̼��Ƶ���������Ϊ83.3%��
������������Ҫ�����йػ�ѧ����ʽ�ļ�������������ļ��㣬�ѶȽ�С��
��2�����ݻ�ѧ����ʽ�ɶ�����̼���������Լ����ʯ��ʯ��̼��Ƶ����������������ʯ��ʯ��̼��Ƶ�����������
����⣺��1�����������غ㶨�ɣ�������̼������Ϊ��150g+12g-157.6g=4.4g��
��2�����ʯ��ʯ��Ʒ��̼��Ƶ�����Ϊx��
CaCO3+2HCl�TCaCl2+H2O+CO2��
100 44
x 4.4g
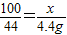 ��x=10g
��x=10g��ʯ��ʯ��̼��Ƶ���������Ϊ��
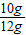 ×100%��83.3%
×100%��83.3%�𣺸�ʯ��ʯ��̼��Ƶ���������Ϊ83.3%��
������������Ҫ�����йػ�ѧ����ʽ�ļ�������������ļ��㣬�ѶȽ�С��

��ϰ��ϵ�д�
�����Ŀ
ijˮ�೧�����ң�Ϊ�˲ⶨij��ɽʯ��ʯ��̼��Ƶ�����������ȡʯ��ʯ��Ʒ������ϡ�������ձ��з�Ӧ������ʯ��ʯ��Ʒ�����ʲ���ϡ���ᷴӦҲ������ˮ�����й�ʵ���������±���
��1�����������غ㶨�ɿ�֪����Ӧ�����ɶ�����̼������Ϊ g��
��2�����ʯ��ʯ��̼��Ƶ�����������
| ��Ӧǰ | ��Ӧ�� | ||
| ʵ �� �� �� |
�ձ���ϡ���� ������ |
ʯ��ʯ��Ʒ ������ |
�ձ������л� ��������� |
| 150g | 12g | 157.6g | |
��2�����ʯ��ʯ��̼��Ƶ�����������