��Ŀ����
����Ŀ��Ϊ�ⶨij������ʯ��������������������С��������ͬѧ��������һ����̼��10g������ʯ��Ʒ��ַ�Ӧ(���ʲ����뷴Ӧ)���������ɵ�������һ����������������Һ��ȫ���գ�����Һ�������뷴Ӧʱ��ı仯��ϵ��ͼ��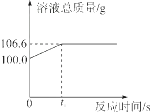
��1��������Ӧ����������̼������Ϊg��
��2������ó�����ʯ��������������������(д���������)
��3����������Ӧ��ʣ���������Ϊmg��д������ó�����ʯ�����������������ı���ʽ(��m��ʾ)��
���𰸡�
��1��6.6
��2��80%
��3��![]() ��100%
��100%
����������1����ͼ��֪����Ӧ����������̼������Ϊ106.6-100.0=6.6g��
��2���������ʯ��������������Ϊx��
3CO+Fe2O3![]() 2Fe+3CO2
2Fe+3CO2
160 3��44
x 6.6g![]() ��
�� ![]()
��ã�x=8.0g
��Ʒ������������������Ϊ��![]() ��100%=80%��
��100%=80%��
�𣺸���Ʒ������������������Ϊ80%��
��3���������ʯ����������������Ϊy���������ʲ���Ӧ��Ҳ���������Ĺ��壬����m�а�����10g��Ʒ�����ʣ�������m��ȥ���ʾ���������������
10gy��![]() ��100%=m-��1-y����10g
��100%=m-��1-y����10g
��ã�y=![]() ��100%.
��100%.
�ʴ�Ϊ����1��6.6g����2��80%����3��![]() ��100%.
��100%.
��1�����������غ㶨�ɣ���106.6g��ȥ100g��Ϊ������̼��������
��2��д��CO��Fe2O3��Ӧ�Ļ�ѧ��Ӧ����ʽ��Ȼ����ݶ�����̼�������������������������������������������������=![]()
��100%��ɣ�
��3����������������Ԫ�ص��������������10g������ʯ��Ʒ��������������ֵ�͵��ڷ�Ӧ��ʣ���������m��ȥ���ʵ�������������ϵʽ�����������m��ʾ�ı���ʽ��

 ���ɿ��õ�Ԫ������ĩר����100��ϵ�д�
���ɿ��õ�Ԫ������ĩר����100��ϵ�д�