��Ŀ����
����Ŀ����ͼ��ʾΪʵ�����г����������Ʊ����������ռ�������ʵ��IJ�����������װʵ��װ��ʱ�����ظ�ѡ����������ijѧУ������ѧʵ��̽���С���ͬѧ����������ɸ��Ե�̽��ʵ�顣
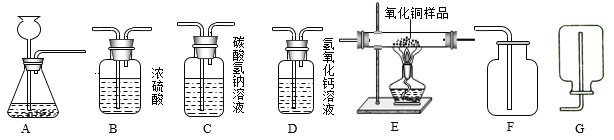
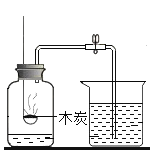
��1����һ���ͬѧ��ʯ��ʯ��ϡ����Ϊԭ�ϣ���ʵ�����Ʊ����ռ����﴿���Ķ�����̼���壬����Ҫ�����ʵ��װ�á�����������������װ�õ������ԡ�����ʾ������ӷ���������HCl������ñ���̼��������Һ���գ�
����ѡ����������˳��Ϊ_____����д���������ĸ����
��д��������ȡ������̼�Ļ�ѧ��Ӧ����ʽ_____��
��д����pH��ֽ����װ��A��ʣ��Һ�����ȵľ��巽��Ϊ_____��
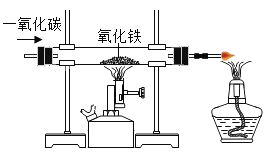
��2���ڶ����ͬѧ����CO����������ˮ������CO2���ʣ����ⶨij����ͭ��Ʒ��CuO����������������ΪCu����ȡ5g����Ʒ��ʵ��ʱ����������˳��Ϊ��������D1��B��E��D2��β������������֪��CuO+CO![]() Cu+CO2����ش��������⣺
Cu+CO2����ش��������⣺
��װ��D1������Ϊ_____��
��ʵ��С���ø�װ�÷�Ӧǰ��������仯�������£�
װ�� | D1 | B | E | D2 |
��Ӧǰ�������仯 | +0.11g | +0.18g | -0.64g | +1.76g |
����ʵ�����ݣ���������Ʒ��CuO����������Ϊ_____%����ȷ��1%����
���𰸡�ACBF CaCO3+2HCl=CaCl2+H2O+CO2�� �ò�����պȡA��Һ����pH��ֽ�ϣ�����ʾ����ɫ�����ɫ�����գ�����pHֵ ����CO2���� 64%
��������
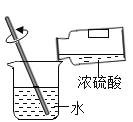
��1������ʵ�����Ʊ����ռ����﴿���Ķ�����̼���壬��ѡ����������˳��Ϊ��ͨ��A��ȡ������̼��ʵ�����ô���ʯ��ϡ���ᷴӦ��ȡ������̼����Ӧ��������Ҫ���ȣ�������ͨ��Cװ�ó�ȥ�Ȼ������壨HCl������ñ���̼��������Һ���գ�����ͨ��Bװ�ó�ȥˮ������Ũ��������ˮ�ԣ������ͨ��Fװ�ÿ����ռ�������Ķ�����̼��������̼�ܶȴ��ڿ����������ACBF��
��ʵ�����ô���ʯ��ϡ���ᷴӦ��ȡ������̼������ʯ����Ҫ�ɷ�̼��ƺ�ϡ���ᷴӦ�Ļ�ѧ����ʽΪ��CaCO3+2HClCaCl2+H2O+CO2�������CaCO3+2HCl=CaCl2+H2O+CO2����
����pH��ֽ����װ��A��ʣ��Һ�����ȵľ��巽��Ϊ���ò�����պȡ����Һ����pH��ֽ�ϣ�����ʾ����ɫ�����ɫ�����գ�����pHֵ������ò�����պȡA��Һ����pH��ֽ�ϣ�����ʾ����ɫ�����ɫ�����գ�����pHֵ��
��2����������������Һ���������̼��Ӧ��װ��D1�������dz�ȥ����CO�л��е�����CO2���������CO2���壻
��Eװ����������С0.64g��˵������ͭ����Ԫ�ص�������0.64g��������ͭ������Ϊ��0.64g��![]() =3.2g������Ʒ��CuO����������Ϊ��
=3.2g������Ʒ��CuO����������Ϊ��![]() ��100%=64%�����64%��
��100%=64%�����64%��

 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�����Ŀ��ij�����������Ĵ����Ʒ�к��������Ȼ������ʣ����Ʒ��װ����ע����̼���ơ�93%��Ϊ�ⶨ�ò�Ʒ�к�̼���Ƶ���������������������ʵ�飺ȡ12.0g������Ʒ�����ձ��У��Ƶ��ձ�����ʢ������Ʒ��������Ϊ158.0g���ٰ�120gϡ����ƽ���ֳ��ķ����μ�����Ʒ�У�ÿ�ξ���ַ�Ӧ��ʵ�����ݼ�¼���£�
��������Ĵ��� | ��һ�� | �ڶ��� | ������ | ���Ĵ� |
ϡ���������/g | 30 | 30 | 30 | 30 |
�ձ�����ʢ����������/g | 186.2 | 214.4 | 243.6 | 273.6 |
����ݴ˷������㣺
��1����_____�μ����ϡ������ȫ��Ӧ�ˡ�
��2���ò�Ʒ��̼���Ƶ����������Ƿ�ϸ�___��Ҫ��д��������̣������ȷ��0.1%��