��Ŀ����
����Ŀ��ij��ѧ��ȤС���ͬѧ��ʵ����������������Ϊ8��������������Һ��������ⶨijϡ���������ʵ�����������
��1������200g��������Ϊ8��������������Һ��
�ټ��㣺��Ҫ�������ƹ��������Ϊ g��ˮ�����Ϊ mL(ˮ���ܶȽ��ƿ���1g��cm3)��
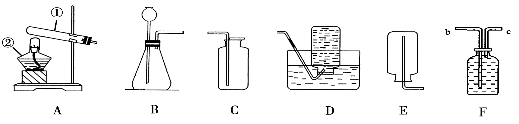
�ڳ���������������ƽƽ�⣬��һ���ձ�����������ƽ�� �̣�������������Ȼ�� (���������Ⱥ�˳��ѡ����ĸ)��ֱ����ƽƽ�⡣
A�����������ƹ�������ձ���
B������Ҫ�������롢�ƶ�����
�ò��������ձ�������ֽ�����������Ƶ�ԭ���� ��
���ܽ⣺����Ͳ��ȡ�����ˮ������ʢ���������ƹ�����ձ�����裬ʹ���ܽ⣬����ȴ�����¡�
�ܰ���õ���Һװ���Լ�ƿ��������Ƥ�������ϱ�ǩ��

���𰸡���16 184 �� �� BA ��NaOH�и�ʴ��
��
����������Һ |
8% |
��������
�����������1������200g��������Ϊ8��������������Һ���ټ��㣺��Ҫ�������ƹ��������Ϊ200g��8%=16g��ˮ�����Ϊ��200g16g����1g��cm3)=184mL(ˮ���ܶȽ��ƿ���1g��cm3)���ڳ���������������ƽƽ�⣬����������ƽ��ʹ�÷������������롱���г������ʽ�һ���ձ�����������ƽ�����̣�������������Ȼ�� B������Ҫ�������롢��A�����������ƹ�������ձ��У��ƶ�����ֱ����ƽƽ�⡣�ò��������ձ�������ֽ�����������Ƶ�ԭ����NaOH�и�ʴ�ԣ����ܽ⣺����Ͳ��ȡ�����ˮ������ʢ���������ƹ�����ձ�����裬ʹ���ܽ⣬����ȴ�����¡��ܰ���õ���Һװ���Լ�ƿ��������Ƥ�������ϱ�ǩ��

 ��Уͨ��֤��Ч��ҵϵ�д�
��Уͨ��֤��Ч��ҵϵ�д�����Ŀ����ȥ���������е��������ʣ���ѡ�õ��Լ���������������ȷ����
ѡ�� | ���ʣ�������Ϊ���ʣ� | �Լ� | �������� |
A | HCl (CO2) | ����NaOH��Һ | ϴ�� |
B | NaCl (Na2CO3) | ����ϡ���� | �������ᾧ |
C | CaO (CaCO3) | ����ˮ | �ܽ⡢���ˡ����� |
D | ϡ���� (ϡ����) | �������ᱵ��Һ | ���� |