��Ŀ����
����Ŀ���ҹ��Ƽҵ������������°�̽�������ˡ������Ƽ���������������漰����Ҫ��ѧ��Ӧ���£�
��NH3��CO2��X===NH4HCO3
��NH4HCO3��NaCl===NH4Cl��NaHCO3��
��2NaHCO3![]() Na2CO3��H2O��CO2��
Na2CO3��H2O��CO2��
��ش��������⣺
(1)��Ӧ����X�Ļ�ѧʽΪ________��
(2)��ȥ����Na2CO3��ĩ��������NaHCO3�ķ�����____________��
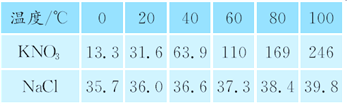
(3)��ҵ�����к����Ȼ��ƣ�ȡ55 g��ҵ��������м���269.5 gϡ���ᣬǡ����ȫ��Ӧ������22 g������̼����
�ٹ�ҵ������̼���Ƶ�����������(������������0.1%) ___________.
�ڷ�Ӧ����Һ�����ʵ�����������____________.
���𰸡� H2O ���������� 96.4% 20%
�����������⿼���������غ㶨�ɺ���ȡ������Ϣ�ó��������ʲ�������г��ӣ������˷���ʽ�ļ��㣬ע������Ӧ����Һ��������������ʱ��Ҫע����Ʒ�е������Ƿ�����Һ�����ʵ�һ������
��1�����ݻ�ѧ��Ӧǰ��ԭ���������Ŀ���䣬��Ӧǰ��������N��H��C��OԪ�ص�ԭ�Ӹ����ֱ�Ϊ�� 1�� 3��1�� 2������Ӧ��������N��H��C��OԪ�ص�ԭ�Ӹ����ֱ�Ϊ�� 1�� 5�� 1�� 3����Ӧ��X��һ�������к���2��Hԭ�Ӻ�1��Oԭ�����ԣ�xΪH2O��
��2������NaHCO3�����ֽ��̼���ơ�ˮ�Ͷ�����̼�����ԣ���ȥ����Na2CO3��ĩ��������NaHCO3�ķ����ǽ��������ȣ�
��3����55g��ҵ������̼���Ƶ�����Ϊx
Na2CO3+2HCl=2NaCl+H2O+CO2��
106 117 44
x y 22g
![]()
![]()
��ã�x=53g
��ҵ������̼���Ƶ���������Ϊ![]() ��100%��96.4%
��100%��96.4%
![]() y=58.5g
y=58.5g
��Ӧ����Һ�������Ȼ��Ƶ���������=![]() ��100%=20%��
��100%=20%��