��Ŀ����
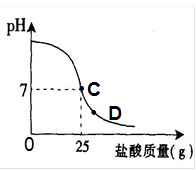
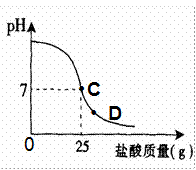
��8�֣�Ϊ�ⶨ������������Ϊ32%�������ʵ������������С��ʵ��ʱ�����ձ��м���20g40%������������Һ������μ�������ᣬ�ⶨ������������������ձ�����ҺpH�Ĺ�ϵ��ͼ��
��1�� ��������������Һ�����ʵ�����Ϊ____________g��
��2�� �����濴����ͼ��Ϣ��ش���������
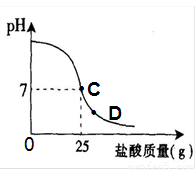
�ٵ��μ����ᵽC��ʱ�������ĵ����������ʵ������Ƕ��٣�
�ڸ������ʵ�����������Ƕ��٣�(������0.1%)
�۵���������Һ�������������ı��ԭ���ǣ� ��
�ܵ��μ����ᵽͼ����D��ʱ���ձ�����Һ�������� �����ѧʽ��
��1��8g����1�֣�
��2����7.3�ˣ�1�֣� ��29. 2% (2��)
�������лӷ��� ����1�֣� ��HCl��NaCl ����2�֣���1�֣�
����������1��NaOH������=20g��40%=8g
��2����C��ʱ��PH=7˵���������ƺ�����ǡ����ȫ����20������������Һ��25������ǡ����ȫ��Ӧ���������е����ʵ�������X��
NaOH + HCl = NaCl + H2O
40 36.5
8�� X
40��36.5 ��8g��X ��ã�X=7.3g
����������ʵ���������7.3g/25g��100%�T29.2%��
��������лӷ��ԣ�������Һ���е��������٣��ܼ����䣬���ϡ�����Ũ����ϡ��
�ܵ���D��ʱ����Һ������˵�������Ѿ���������ʱ��Һ�����������ɵ�NaCl�����й�����HCl��

 ��Ч���ܿ�ʱ��ҵϵ�д�
��Ч���ܿ�ʱ��ҵϵ�д�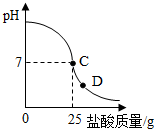 ������������������ձ�����ҺpH�Ĺ�ϵ��ͼ��
������������������ձ�����ҺpH�Ĺ�ϵ��ͼ��