��Ŀ����
����Ŀ�������dz��л�ѧ������ʵ��װ��ͼ����ش��й����⣺
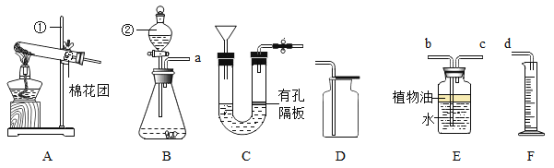
��1��ʵ�����ø��������ȡ������ѡ�õķ���װ����____________������ĸ�����÷�Ӧ�Ļ�ѧ��ʽΪ____________��
��2�����ڶ�����̼��
��ʵ���Ҽ���CO2����Ӧ�Ļ�ѧ����ʽΪ_________________________________��
������Cװ���ƶ�����̼���ŵ���____________________________________��
������B��E��Fװ����ϲⶨ���ɶ�����̼���������װ�ýӿ�˳��Ϊa_____d����b��c˳����E�в���ֲ���ͻᵼ�²ⶨ���____________���ƫ����ƫС�����䡱����
���𰸡�A ![]()
![]() ���ڿ��Ʒ�Ӧ�ķ�����ֹͣ cb ƫС
���ڿ��Ʒ�Ӧ�ķ�����ֹͣ cb ƫС
��������
��1��ʵ�����ø��������ȡ�������ǹ�������ͷ�Ӧ���ʿ�ѡ�õķ���װ����A���÷�Ӧ�Ļ�ѧ����ʽΪ2KMnO4![]() K2MnO4+MnO2+O2�������A��2KMnO4
K2MnO4+MnO2+O2�������A��2KMnO4![]() K2MnO4+MnO2+O2����
K2MnO4+MnO2+O2����
��2����ʵ���Ҽ���CO2��ʹ�õ��dz����ʯ��ˮ����Ӧ�Ļ�ѧ����ʽΪCa��OH��2+CO2=CaCO3��+H2O�����Ca��OH��2+CO2=CaCO3��+H2O��
������Cװ���ƶ�����̼�����ܿ��Ʒ�Ӧ�ķ�����ֹͣ������ܿ��Ʒ�Ӧ�ķ�����ֹͣ��
������B��E��Fװ����ϲⶨ���ɶ�����̼���������װ�ýӿ�˳��Ϊacbd����E�в���ֲ���ͣ��������̼������ˮ���ᵼ�²ⶨ���ƫС�����cb��ƫС��

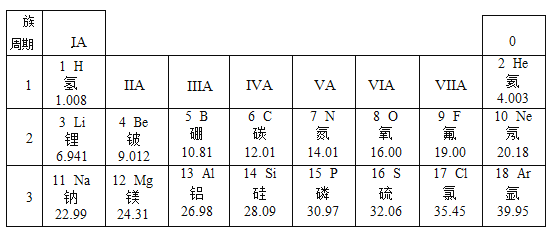
����Ŀ����ͼ��Ԫ�����ڱ���һ����,��ش��������
��һ���� | 1 H | 2 He | ||||||
�ڶ����� | 3 Li | 4 Be | 5 B | 6 C | 7 N | 8 O | 9 F | 10 Ne |
�������� | 11 Na | 12 Mg | 13 A1 | 14 Si | 15 P | 16 S | 17 CI | 18 Ar |
��1����Ԫ������________(���������������ǽ�����)Ԫ�أ���ԭ���ڻ�ѧ��Ӧ����_______(�����õ�������ʧȥ��)���ӡ�
��2����Ԫ������Ԫ�ػ�ѧ�������Ƶ�ԭ����__________��
��3��д��12��Ԫ����ɵĵ��ʺ�8��Ԫ����ɵĵ��ʷ�Ӧ�Ļ�ѧ����ʽ_________��