��Ŀ����
��2002?���ݣ�������������һλС������1����50.0�˲�����ʳ����Ʒ�����ʲ�����ˮ������360.0��ˮ�У���ֽ��裬����Ʒ�е��Ȼ���ȫ���ܽ����ˣ��������������ˮ������ʧ����ȡ��Һ117.0�ˣ������м�����������������Һ���õ�28.7�˰�ɫ����������
����ȡ��117.0����Һ�����ʵ�����������
��ʳ����Ʒ���Ȼ��Ƶ�����������
��2����26.0�˲������Ȼ�����ĩ�����ʲ�����ˮҲ���μӷ�Ӧ������һ������������Һ�У�ǡ����ȫ��Ӧ����Ӧ��õ�������������Ϊ1-2.0%����Һ97.5�ˣ����㣺
�ٷ�Ӧ����Һ�����ʵ������Ƕ��ٿˣ�
�ڲ������Ȼ�����ĩ�к��Ȼ��������������Ƕ��٣�
�۷�Ӧǰ��������Һ�������Ƕ��ٿˣ�
���𰸡���������1���ٸ������������Ȼ��Ʒ�Ӧ�Ļ�ѧ����ʽ�����ɳ������������г�����ʽ���Ϳɼ�������뷴Ӧ��NaCl����������������������Ȼ����ݡ�������������=����������Һ����×100%�����㼴�ɣ�
�ڸ��ݡ��ܼ�����÷�ܼ��������������Һ�������ٸ��ݡ���Һ����×10%�������ʳ����Ʒ���Ȼ��Ƶ�������Ȼ���������������ʽ���㼴�ɣ�
��2���ٸ�����������=��Һ����×���������������㼴�ɣ�
�ڸ���Na2SO4��BaCl2��Ӧ�Ļ�ѧ����ʽ�Լ���1���м���������ɵ��Ȼ��Ƶ����������ɼ������������Ȼ��������������ɳ�����������Ȼ���������������ʽ���㼴�ɣ�
�۸��������غ㶨�ɣ��ڻ�ѧ��Ӧ�У��μӷ�Ӧǰ�����ʵ������ܺ͵��ڷ�Ӧ�����ɸ����ʵ������ܺͣ���Ӧǰ��������Һ����=��Ӧ��NaCl��Һ������+BaSO4����������-�������BaCl2��������
����⣺��1��������뷴Ӧ��NaCl������Ϊx��
AgNO3+NaCl�TAgCl��+NaNO3
58.5 143.5
x 28.7g
��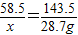
��֮�ã�x=11.7g��
��ȡ��117����Һ�����ʵ���������Ϊ��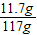 ×100%=10%��
×100%=10%��
����Һ��������Ϊ��360g÷��1-10%��=400g��
ʳ����Ʒ���Ȼ��Ƶ�����Ϊ��400g×10%=40g��
ʳ����Ʒ���Ȼ��Ƶ���������Ϊ��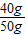 ×100%=80%��
×100%=80%��
�𣺢���ȡ��117����Һ�����ʵ���������Ϊ10%��
��ʳ����Ʒ���Ȼ��Ƶ���������Ϊ80%��
��2����97.5g×12.0%=11.7g��
�����������Ȼ���������Ϊx�����ɳ���������Ϊy��
Na2SO4+BaCl2=2NaCl+BaSO4��
208 117 233
x 11.7g y
�� ��
��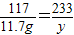 ��
��
��֮�ã�x=20.8g��y=23.3g��
��������Ȼ����������ٷֺ���Ϊ��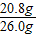 ×100%=80%��
×100%=80%��
�۷�Ӧǰ��������Һ������=97.5g+23.3g-11.7g=109.1g��
�𣺢ٷ�Ӧ����Һ����������Ϊ11.7g��
�ڻ�������Ȼ����������ٷֺ���Ϊ80%��
�۷�Ӧǰ��������Һ����Ϊ109.1g��
������������Ҫ����ѧ�����û�ѧ����ʽ����������������ʽ���м��������������ѧ�����������غ㶨�ɺͻ�ѧ����ʽ�Լ�����������ʽ���м������������
�ڸ��ݡ��ܼ�����÷�ܼ��������������Һ�������ٸ��ݡ���Һ����×10%�������ʳ����Ʒ���Ȼ��Ƶ�������Ȼ���������������ʽ���㼴�ɣ�
��2���ٸ�����������=��Һ����×���������������㼴�ɣ�
�ڸ���Na2SO4��BaCl2��Ӧ�Ļ�ѧ����ʽ�Լ���1���м���������ɵ��Ȼ��Ƶ����������ɼ������������Ȼ��������������ɳ�����������Ȼ���������������ʽ���㼴�ɣ�
�۸��������غ㶨�ɣ��ڻ�ѧ��Ӧ�У��μӷ�Ӧǰ�����ʵ������ܺ͵��ڷ�Ӧ�����ɸ����ʵ������ܺͣ���Ӧǰ��������Һ����=��Ӧ��NaCl��Һ������+BaSO4����������-�������BaCl2��������
����⣺��1��������뷴Ӧ��NaCl������Ϊx��
AgNO3+NaCl�TAgCl��+NaNO3
58.5 143.5
x 28.7g
��
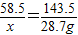
��֮�ã�x=11.7g��
��ȡ��117����Һ�����ʵ���������Ϊ��
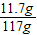 ×100%=10%��
×100%=10%������Һ��������Ϊ��360g÷��1-10%��=400g��
ʳ����Ʒ���Ȼ��Ƶ�����Ϊ��400g×10%=40g��
ʳ����Ʒ���Ȼ��Ƶ���������Ϊ��
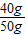 ×100%=80%��
×100%=80%���𣺢���ȡ��117����Һ�����ʵ���������Ϊ10%��
��ʳ����Ʒ���Ȼ��Ƶ���������Ϊ80%��
��2����97.5g×12.0%=11.7g��
�����������Ȼ���������Ϊx�����ɳ���������Ϊy��
Na2SO4+BaCl2=2NaCl+BaSO4��
208 117 233
x 11.7g y
��
 ��
��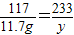 ��
����֮�ã�x=20.8g��y=23.3g��
��������Ȼ����������ٷֺ���Ϊ��
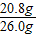 ×100%=80%��
×100%=80%���۷�Ӧǰ��������Һ������=97.5g+23.3g-11.7g=109.1g��
�𣺢ٷ�Ӧ����Һ����������Ϊ11.7g��
�ڻ�������Ȼ����������ٷֺ���Ϊ80%��
�۷�Ӧǰ��������Һ����Ϊ109.1g��
������������Ҫ����ѧ�����û�ѧ����ʽ����������������ʽ���м��������������ѧ�����������غ㶨�ɺͻ�ѧ����ʽ�Լ�����������ʽ���м������������

��ϰ��ϵ�д�
�����Ŀ