��Ŀ����
��2011?���£���ѧ�о���С��������к�ʵ�飮
��ͬѧ������ʦָ���£����������ƹ�������100g������������Ϊ10%������������Һ��
��1���ܽ�ʱ��Ҫ�õ�����������������
��2�����в����ᵼ�����Ƶ�����������Һ���ʵ���������ƫ�͵���
A���������ƹ����к��в�����ˮ������
B����ˮϴ�����ȼ��δ�����ֱ����������
C������Ͳ��ȡˮ�����ʱ���ø��ӵķ�������
��С���ͬѧ��ϡ������������Ƶ�����������Һ�����к�ʵ��ʱ�����������ݲ�����С��ͬѧ�������ɣ�Ҫ����ƿ���ʵ��������ƹ������̽����
[����]�������ƹ�����¶�ڿ����в������գ�������������ж�����̼��Ӧ�����ʣ�д���÷�Ӧ�Ļ�ѧ����ʽ
[�������]����һ��������������Ʒ���ֱ��ʣ�
�������������������Ʒȫ�����ʣ�
[��������]����֪��Ӧ��CaCl2+Na2CO3�T2NaCl+CaCO3��
�ڲ���������Һ��pH���±���
[����ʵ��]���������ǹ�ͬ��ɣ����ش����������⣺
[��չ]�о���С��ͬѧ�ڲ�����еμӵ��Լ�����ָʾ���⣬��������
[��˼]NaOH����Ӧ
��ͬѧ������ʦָ���£����������ƹ�������100g������������Ϊ10%������������Һ��
��1���ܽ�ʱ��Ҫ�õ�����������������
�����ܽ�
�����ܽ�
����2�����в����ᵼ�����Ƶ�����������Һ���ʵ���������ƫ�͵���
A��B
A��B
������ţ�A���������ƹ����к��в�����ˮ������
B����ˮϴ�����ȼ��δ�����ֱ����������
C������Ͳ��ȡˮ�����ʱ���ø��ӵķ�������
��С���ͬѧ��ϡ������������Ƶ�����������Һ�����к�ʵ��ʱ�����������ݲ�����С��ͬѧ�������ɣ�Ҫ����ƿ���ʵ��������ƹ������̽����
[����]�������ƹ�����¶�ڿ����в������գ�������������ж�����̼��Ӧ�����ʣ�д���÷�Ӧ�Ļ�ѧ����ʽ
2NaOH+CO2�TNa2CO3+H2O
2NaOH+CO2�TNa2CO3+H2O
��[�������]����һ��������������Ʒ���ֱ��ʣ�
�������������������Ʒȫ�����ʣ�
[��������]����֪��Ӧ��CaCl2+Na2CO3�T2NaCl+CaCO3��
�ڲ���������Һ��pH���±���
| ����Һ | CaCl2��Һ | Na2CO3��Һ | NaCl��Һ |
| pH | ����7 | ����7 | ����7 |
| ʵ�鲽�� | ʵ������ | ʵ����� |
| ����һ��ȡ������Ʒ���Թ��У� ������ˮ��ȫ���ܽ⣬�� ��CaCl2��Һ����������ַ� Ӧ���ã� | ��ɫ���� | ��Ʒ��һ������ ̼���� ̼���� |
| ����һ��ȡ����һ��ַ�Ӧ�� �������ϲ���Һ���Թ��У��� ����ɫ��̪��Һ���� | ��� ��� | �������ʵ��ó� ����һ������ |
����ͭ��Һ
����ͭ��Һ
�����[��˼]NaOH����Ӧ
�ܷ�
�ܷ�
���森��������ͬѧ������ʦָ���£����������ƹ�������100g������������Ϊ10%������������Һ��
��1���ܽ�ʱ��Ҫ�õ����������������� �����ܽ⣻
��2�����в����ᵼ�����Ƶ�����������Һ���ʵ���������ƫ�͵ģ�����Ͳ��ȡˮʱ������ˮ��̶ȶ�������ˮʱ�����ӿ̶ȣ������ˮƫ�٣�
���ֱ��ʣ�˵��һ�����������Ʒ����˷�Ӧ���������ƺͿ����еĶ�����̼����̼���ƺ�ˮ����Ʒ���������ʵ�飺̼���ƺ��Ȼ�������̼��Ƴ������Ȼ��ƣ���Ӧ����Һ���������Ȼ��ƺ��Ȼ��ƣ�ʣ�ࣩ������ͼ����֪���Ȼ�����Һ���Ȼ�����Һ��pH������7����̼���Ƴ�����ģ�����ʱ��Һ�������˵����Һ�л����������ƣ�ʵ����ۣ���ɫ����˵����Ʒ����̼���ƣ�˵����Ʒ�����������ƣ�
��չ��������������ͭ���ӷ�Ӧ������ɫ������
��˼���������������ܷⱣ�森
��1���ܽ�ʱ��Ҫ�õ����������������� �����ܽ⣻
��2�����в����ᵼ�����Ƶ�����������Һ���ʵ���������ƫ�͵ģ�����Ͳ��ȡˮʱ������ˮ��̶ȶ�������ˮʱ�����ӿ̶ȣ������ˮƫ�٣�
���ֱ��ʣ�˵��һ�����������Ʒ����˷�Ӧ���������ƺͿ����еĶ�����̼����̼���ƺ�ˮ����Ʒ���������ʵ�飺̼���ƺ��Ȼ�������̼��Ƴ������Ȼ��ƣ���Ӧ����Һ���������Ȼ��ƺ��Ȼ��ƣ�ʣ�ࣩ������ͼ����֪���Ȼ�����Һ���Ȼ�����Һ��pH������7����̼���Ƴ�����ģ�����ʱ��Һ�������˵����Һ�л����������ƣ�ʵ����ۣ���ɫ����˵����Ʒ����̼���ƣ�˵����Ʒ�����������ƣ�
��չ��������������ͭ���ӷ�Ӧ������ɫ������
��˼���������������ܷⱣ�森
����⣺�����������ƹ�������100g������������Ϊ10%������������Һ��
��1���ܽ�ʱ��Ҫ�õ����������������� �����ܽ⣻
��2�����в����ᵼ�����Ƶ�����������Һ���ʵ���������ƫ�͵ģ�
A���������ƹ����к��в�����ˮ�����ʣ����������Ƶ�����ƫС������������ƫ�ͣ�
B����ˮϴ�����ȼ��δ�����ֱ���������ƣ���ˮƫ�࣬������������Һ���ʵ���������ƫ�ͣ�
C������Ͳ��ȡˮʱ������ˮ��̶ȶ�������ˮʱ�����ӿ̶ȣ������ˮƫ�٣�����������Һ���ʵ���������ƫ�ߣ���ѡA��B��
���ֱ��ʣ�˵��һ�����������Ʒ����˷�Ӧ���������ƺͿ����еĶ�����̼����̼���ƺ�ˮ����Ʒ���������ʵ�飺̼���ƺ��Ȼ�������̼��Ƴ������Ȼ��ƣ���Ӧ����Һ���������Ȼ��ƺ��Ȼ��ƣ�ʣ�ࣩ������ͼ����֪���Ȼ�����Һ���Ȼ�����Һ��pH������7����̼���Ƴ�����ģ�����ʱ��Һ�������˵����Һ�л����������ƣ�ʵ����ۣ���ɫ����˵����Ʒ����̼���ƣ�˵����Ʒ�����������ƣ�
��չ��������������ͭ���ӷ�Ӧ������ɫ����������������ͭ��Һ��
��˼���������������ܷⱣ�森
�ʴ�Ϊ����1�������ܽ⣻��2��A��B��
��[����]2NaOH+CO2�TNa2CO3+H2O��
[����ʵ��]��
[��չ]����ͭ��Һ��
[��˼]�ܷ�
��1���ܽ�ʱ��Ҫ�õ����������������� �����ܽ⣻
��2�����в����ᵼ�����Ƶ�����������Һ���ʵ���������ƫ�͵ģ�
A���������ƹ����к��в�����ˮ�����ʣ����������Ƶ�����ƫС������������ƫ�ͣ�
B����ˮϴ�����ȼ��δ�����ֱ���������ƣ���ˮƫ�࣬������������Һ���ʵ���������ƫ�ͣ�
C������Ͳ��ȡˮʱ������ˮ��̶ȶ�������ˮʱ�����ӿ̶ȣ������ˮƫ�٣�����������Һ���ʵ���������ƫ�ߣ���ѡA��B��
���ֱ��ʣ�˵��һ�����������Ʒ����˷�Ӧ���������ƺͿ����еĶ�����̼����̼���ƺ�ˮ����Ʒ���������ʵ�飺̼���ƺ��Ȼ�������̼��Ƴ������Ȼ��ƣ���Ӧ����Һ���������Ȼ��ƺ��Ȼ��ƣ�ʣ�ࣩ������ͼ����֪���Ȼ�����Һ���Ȼ�����Һ��pH������7����̼���Ƴ�����ģ�����ʱ��Һ�������˵����Һ�л����������ƣ�ʵ����ۣ���ɫ����˵����Ʒ����̼���ƣ�˵����Ʒ�����������ƣ�
��չ��������������ͭ���ӷ�Ӧ������ɫ����������������ͭ��Һ��
��˼���������������ܷⱣ�森
�ʴ�Ϊ����1�������ܽ⣻��2��A��B��
��[����]2NaOH+CO2�TNa2CO3+H2O��
[����ʵ��]��
| ʵ�鲽�� | ʵ������ | ʵ����� |
| ����һ��ȡ������Ʒ���Թ��У� ������ˮ��ȫ���ܽ⣬�� ��CaCl2��Һ����������ַ� Ӧ���ã� | ��ɫ���� | ��Ʒ��һ������ ̼���� |
| ����һ��ȡ����һ��ַ�Ӧ�� �������ϲ���Һ���Թ��У��� ����ɫ��̪��Һ���� | ��� | �������ʵ��ó� ����һ������ |
[��˼]�ܷ�
���������շ���������ɵķ�������������һ��Ҫ�����������ԣ����磺���������壮���ճ��û�ѧ��Ӧ��������ص㣮

��ϰ��ϵ�д�
 ��У����ϵ�д�
��У����ϵ�д�
�����Ŀ
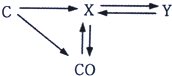 ��2011?���£�����֪ʶ������һ����Ҫ��ѧϰ��������ͼ��̼���ʺͲ���̼�Ļ�������ת��
��2011?���£�����֪ʶ������һ����Ҫ��ѧϰ��������ͼ��̼���ʺͲ���̼�Ļ�������ת��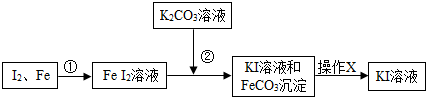
 ��2011?�����ʼ죩���������ƹ����п��ܻ���̼���ƣ��ס��ҡ�����λͬѧ�ֱ�ȡ������ˮ���м��飮
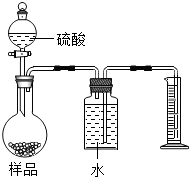
��2011?�����ʼ죩���������ƹ����п��ܻ���̼���ƣ��ס��ҡ�����λͬѧ�ֱ�ȡ������ˮ���м��飮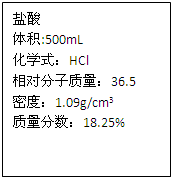 ��2011?�����ʼ죩ʵ���ұ����һƿ���ᣬƿ�ϱ�ǩ�IJ���������ͼ��������ݱ�ǩ���ṩ�����ݽ���������⣺��1��ƿ��������Һ������Ϊ
��2011?�����ʼ죩ʵ���ұ����һƿ���ᣬƿ�ϱ�ǩ�IJ���������ͼ��������ݱ�ǩ���ṩ�����ݽ���������⣺��1��ƿ��������Һ������Ϊ