��Ŀ����
����Ŀ��(9��)Ϊ̽��������ʣ���������ʵ�顣

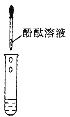
��1����ͼ����ʾ������һ�������������Һ��ɫ��ȥ����Һ�¶� (����������������������������)��(2��)
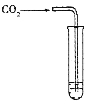
��2����ͼ�Һͱ���ʾ��ͬʱ����֧����CO2����ͬ�Թֱܷ��ڵ������ˮ�� NaOH��Һ�С�һ��ʱ�����Һ������ң�˵��NaOH��CO2�����˷�Ӧ���÷�Ӧ�Ļ�ѧ����ʽΪ_______________(3��)��ʵ�����ҵ�������__________________________(2��)��
��3��ʵ��������з�Һ����������������Һȫ������һ���ձ��У��ٽ���������Һ�������롣ȫ�����������ҺpHΪ3������PH�ӽ�7���ﵽ�ŷű�������������Һ��������Ĺ����У����ձ��ڿɹ۲쵽��������__________________________(2��)
���𰸡���1������
��2��2NaOH+CO2==Na2CO3+H2O ���ж���ʵ�飬֤��CO2��NaOH�����˷�Ӧ
��3����Һ�ȱ�����ɫ���������ݲ���
�������������������1������кͷ�Ӧ��ų�����������NaOH��Һ�м���һ�������������Һ�¶�����
��2��NaOH��CO2������Ӧ��ѧ����ʽ��2NaOH+CO2==Na2CO3+H2O��ʵ�����ҵ������ǣ����ж���ʵ�飬�ų�NaOH�ܽ���ˮ��ʹѹǿ���ͣ�֤��CO2��NaOH�����˷�Ӧ
��3�����ڱ��з�����Ӧ��2NaOH+CO2==Na2CO3+H2O���ʱ�������Һ������ΪNa2CO3������������Һ�������롣ȫ�����������ҺpHΪ3��˵������Һ�к����ᣬ�ʻ��з�Ӧ��Na2CO3+2HCl==2NaCl+H2O+CO2���������ʴ��ձ��ڿɹ۲쵽�������ǣ���Һ�ȱ�����ɫ���������ݲ���

 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�