��Ŀ����
����Ŀ������к�Ρ��Ļ�ѧʽΪMgSO4KClxH2O����һ����ȡ�طʵ���Ҫԭ�ϣ�������ˮ�õ�KCl��MgSO4�Ļ����Һ����ѧ�С�������������ʵ�鷽��������˵������ȷ���ǣ���Է�������MgSO4��120 BaSO4��233 AgCl�� 143.5 KCl��74.5���� ��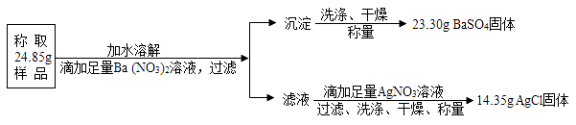
A.�÷����ܹ����������к�Ρ���KCl����������
B.����Ba(NO3)2��Һ��Ϊ����MgSO4��ַ�Ӧ
C.����к�Ρ���ѧʽ��x=2
D.����ʵ�����ݵIJⶨ����ѧУʵ�������������ƽ�������
���𰸡�C,D
��������A��������������������Һ���ܽ�������ת��Ϊ�������ɳ���������������Ȼ��ص�������Ȼ�������Ȼ��ص�����������A���������⣻
B����������Ba��NO3��2��Һ��Ϊ����MgSO4��ַ�Ӧ���Ӷ���������þ��������B���������⣻
C�������ṩ�����ݣ��ɼ��������þ���Ȼ��ص�������Ȼ����ݻ�ѧʽ�������ѧʽ��x��ֵ��
�����Ʒ��MgSO4������ΪX
MgSO4+Ba��NO3��2= | BaSO4��+Mg��NO3��2 |
120 | 233 |
x | 23.30g |
![]() ��
�� ![]() ��ã�X=12.00g��
��ã�X=12.00g��
����Ʒ��MgSO4������Ϊ12.00g
�����Ʒ��KCl������ΪY
AgNO3+KCl= | AgCl��+KNO3 |
74.5 | 143.5 |
y | 14.35g |
![]() ��
�� ![]() ��ã�Y=7.45g��
��ã�Y=7.45g��
����Ʒ��KCl������Ϊ7.45g��
ˮ������Ϊ24.85g-12.00g-7.45g=5.4g
���ݡ���к�Ρ��Ļ�ѧʽ
MgSO4KClxH2O |
120 18x |
12.00 5.4g |
![]() ��
�� ![]() ��ã�x=3��C�������⣻
��ã�x=3��C�������⣻
D����Ϊ������ƽֻ�ܾ�ȷ��0.1g������Ŀ�е����ݾ�ȷ��0.01g��D�������⣻
���Դ��ǣ�CD��
�����㾫����������Ҫ�����˸��ݻ�ѧ��Ӧ����ʽ�ļ�������֪ʶ�㣬��Ҫ���ո����ʼ�������=ϵ������Է�������֮�Ȳ�����ȷ�����⣮

 ȫ�ܲ����ĩС״Ԫϵ�д�
ȫ�ܲ����ĩС״Ԫϵ�д�