��Ŀ����
(1)���ǵ������벻����Һ�����粡����ʱ��Ҫ��Һ��������ʱҪ�����ϣ���ͥ���Ҫ�õ�ϴ�Ӽ���
��ҽ��������ˮ�У����ʵĻ�ѧʽ��____��
��ˮ��pH��7��̼�����ϵ�pH___7������ڡ�����С�ڡ����ڡ���������̼������ʱҪͨ����ѹ�ķ�����ʹ�϶�Ķ�����̼�ܽ���ˮ���ʱ������Ӧ�Ļ�ѧ����ʽΪ______________��
�ۼ��á�����顱����Ҫ�ɷ�Ϊ���ᣬ������顱�������ڴ���ʯ���������ǣ�д���йط�Ӧ�Ļ�ѧ����ʽ��_____________��
(2)��ͼ�Ǽס��ҡ����������ʵ��ܽ�����ߡ�
��ҽ��������ˮ�У����ʵĻ�ѧʽ��____��
��ˮ��pH��7��̼�����ϵ�pH___7������ڡ�����С�ڡ����ڡ���������̼������ʱҪͨ����ѹ�ķ�����ʹ�϶�Ķ�����̼�ܽ���ˮ���ʱ������Ӧ�Ļ�ѧ����ʽΪ______________��
�ۼ��á�����顱����Ҫ�ɷ�Ϊ���ᣬ������顱�������ڴ���ʯ���������ǣ�д���йط�Ӧ�Ļ�ѧ����ʽ��_____________��
(2)��ͼ�Ǽס��ҡ����������ʵ��ܽ�����ߡ�
�뿴ͼ�ش����⡣
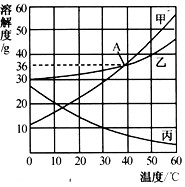
��20��ʱ���ס��ҡ����������ʵ��ܽ���ɴ�С��˳��Ϊ____��
��ͼ��A���ʾ��������____��
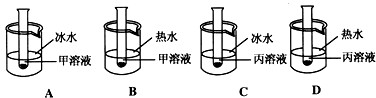
��������ʱ�����ֱ�ʢ�мס���������Һ���Թܸ���֧���ֱ����ʢ�б�ˮ����ˮ���ձ��С����ݼס��������ʵ��ܽ�����ߣ��ж���ͼ�Թ�����ʾ������ȷ����____������ĸ��ţ�����������____��
��20��ʱ���ס��ҡ����������ʵ��ܽ���ɴ�С��˳��Ϊ____��
��ͼ��A���ʾ��������____��
��������ʱ�����ֱ�ʢ�мס���������Һ���Թܸ���֧���ֱ����ʢ�б�ˮ����ˮ���ձ��С����ݼס��������ʵ��ܽ�����ߣ��ж���ͼ�Թ�����ʾ������ȷ����____������ĸ��ţ�����������____��
(1)��NaCl����С�ڣ�CO2+H2O==H2CO3����CaCO3+2HCl==CaCl2+H2O+CO2��
(2)����>��>��
��40��ʱ���ס��������ʵ��ܽ����ȣ���Ϊ36g��
��A��D���ᾧ
(2)����>��>��
��40��ʱ���ס��������ʵ��ܽ����ȣ���Ϊ36g��
��A��D���ᾧ

��ϰ��ϵ�д�
�����Ŀ
 11����ѧԴ�����ͬʱ�ַ���������ճ������������ѧ�������ǵ������벻����ѧ���ش��������⣮
11����ѧԴ�����ͬʱ�ַ���������ճ������������ѧ�������ǵ������벻����ѧ���ش��������⣮
