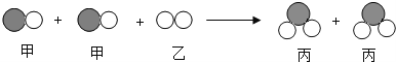��Ŀ����
����Ŀ�����Ȳ�Ʒ�Ѿ��㷺Ӧ���������У���ѧ��ȤС���ijƷ�Ƶ���ů�����������˳�����֤��̽����

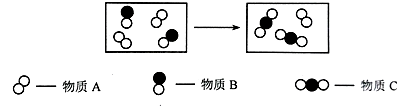
��ʵ��һ����ͼ����ȤС����֤��ɫ��ĩ�к���̼�۵�ʵ��װ��ͼ��
�������ۡ��ձ��ڱ�ʯ��ˮ����ǣ������ů�������к��л���̿��
����˼���ۡ�(1)������֤��ɫ��ĩ�Ƿ���̼�۵ķ����Ƿ���У�__________(����������������������)��������_________��
(2)����ԭ����������������ʴ���ų����������е��Ȼ��Ƶ�������___________���ڴ˱仯��_________��ת��Ϊ_________�ܡ�
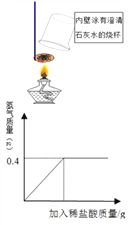
��ʵ������ⶨ��һ����ů�����������ĺ�����һ����ů������(50 g)��ȥ����װ�������з�ĩ������Թܣ��÷�Һ©��������������Ϊ10%ϡ���������ٲ�������Ϊֹ��ͬʱ�������ɵ����塣����ϡ����������������������ϵ����ͼ��ʾ���������ů��������������������Ϊ__��(����Ҫд����)
���𰸡� ������ ��Ϊ�ƾ�ȼ�գ�Ҳ�ܲ���������̼���壬Ҳ��ʹʯ��ˮ����� �ӿ������������ ��ѧ�� ���� 22.4%
�����������⿼����������ʴԭ���ø��ݻ�ѧ����ʽ���㡣
����˼���ۡ�(1) ��֤��ɫ��ĩ�Ƿ���̼�۵ķ��������У���Ϊ�ƾ�ȼ�գ�Ҳ�ܲ���������̼���壬Ҳ��ʹʯ��ˮ����ǣ�
(2) ����ԭ����������������ʴ���ų����������е��Ȼ��Ƶ������Ǽӿ�����������ʣ��ڴ˱仯�л�ѧ��ת��Ϊ���ܣ�
��ʵ���������ͼʾ��֪��Ӧ��������0.4g������
�裺����0.4g��������Ҫ��������Ϊx
Fe+2HCl=FeCl2+H2��
56 2
X 0.4g
![]()
X=11.2g
������������Ϊ=![]() ��100%=22.4%
��100%=22.4%

 �����������һ��һ��ϵ�д�
�����������һ��һ��ϵ�д�