��Ŀ����
��7�֣�ˮ����Ҫ����Ȼ��Դ��
��1����ͼ��ʾ��3��ʵ�飬A��ˮ������ �����������ѧ�����仯��B���Թ�1�ڵõ�������Ϊ ��C�о���ˮ�ķ����� ��������
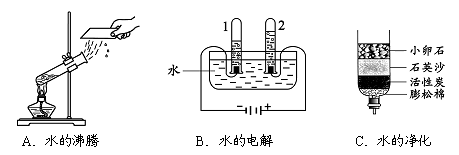
��2����Դˮ����������ˮ��Ҫ������������������Һ������ʱ������Ӧ���۹�������ͼ��
�÷�Ӧ�Ļ�ѧ����ʽΪ___________��D��������Ԫ�صĻ��ϼ���________��
��3�����ֵ�������Ȼˮ��Ca2+��Mg2+�������꣬��ΪӲˮ����Ҫ������������Ca2+��Mg2+������
�����һ���̶��Ͽ������������ã���Ϊ����й����У�����ˮ�е�̼�����[Ca��HCO3��2]�ᷢ�����·�Ӧ��Ca��HCO3��2=CaCO3��+CO2��+H2O,��ˮ�к��н϶��Ca��HCO3��2����й����п��ܹ۲쵽�������� ��
����в��ܱ�������Ӳˮͨ����ѧ��Ӧ�������������Na2CO3���dz��õ�ˮ��������̼������Һ��ˮ�е��Ȼ��Ʒ�Ӧ����̼��ƺ�һ���������Ʒ����Ӧ�Ļ�ѧ����ʽΪ ��
��1����ͼ��ʾ��3��ʵ�飬A��ˮ������ �����������ѧ�����仯��B���Թ�1�ڵõ�������Ϊ ��C�о���ˮ�ķ����� ��������

��2����Դˮ����������ˮ��Ҫ������������������Һ������ʱ������Ӧ���۹�������ͼ��

�÷�Ӧ�Ļ�ѧ����ʽΪ___________��D��������Ԫ�صĻ��ϼ���________��
��3�����ֵ�������Ȼˮ��Ca2+��Mg2+�������꣬��ΪӲˮ����Ҫ������������Ca2+��Mg2+������
�����һ���̶��Ͽ������������ã���Ϊ����й����У�����ˮ�е�̼�����[Ca��HCO3��2]�ᷢ�����·�Ӧ��Ca��HCO3��2=CaCO3��+CO2��+H2O,��ˮ�к��н϶��Ca��HCO3��2����й����п��ܹ۲쵽�������� ��
����в��ܱ�������Ӳˮͨ����ѧ��Ӧ�������������Na2CO3���dz��õ�ˮ��������̼������Һ��ˮ�е��Ȼ��Ʒ�Ӧ����̼��ƺ�һ���������Ʒ����Ӧ�Ļ�ѧ����ʽΪ ��
��7�֣�ÿ��1��
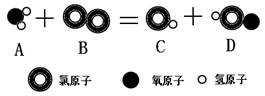
��1��������������H2��������
��2��Cl2+H2O="HCl+HClO" �� +1
��3����ˮ����ǣ�������ð������CaCl2+Na2CO3 =CaCO3��+ 2NaCl
��1��������������H2��������
��2��Cl2+H2O="HCl+HClO" �� +1
��3����ˮ����ǣ�������ð������CaCl2+Na2CO3 =CaCO3��+ 2NaCl
��1��ˮ�����������仯�����ˮ��ʵ���У������ϵ��������������⣻����ˮ��ʵ���У�С��ʯ��ʯӢɳ��������ã�����̿���������ã��ʴ�Ϊ��������������H2�������ˣ�
��2��������ˮ��Ӧ��������ʹ����ᣬ�����������ϼ۵Ĵ�����Ϊ������Ԫ�صĻ��ϼ�Ϊ+1�ۣ��ʴ�Ϊ��Cl2+H2O=HCl+HClO��+1��
��3��̼��������ȷֽ����ɰ�ɫ����̼��ƺ�ˮ�Ͷ�����̼����������ǣ�����ǣ�������ð����̼������Һ��ˮ�е��Ȼ��Ʒ�Ӧ����̼��ƺ��Ȼ��ƣ���ƽ���ɣ��ʴ�Ϊ��ˮ����ǣ�������ð����CaCl2+Na2CO3=CaCO3��+2NaCl
��2��������ˮ��Ӧ��������ʹ����ᣬ�����������ϼ۵Ĵ�����Ϊ������Ԫ�صĻ��ϼ�Ϊ+1�ۣ��ʴ�Ϊ��Cl2+H2O=HCl+HClO��+1��
��3��̼��������ȷֽ����ɰ�ɫ����̼��ƺ�ˮ�Ͷ�����̼����������ǣ�����ǣ�������ð����̼������Һ��ˮ�е��Ȼ��Ʒ�Ӧ����̼��ƺ��Ȼ��ƣ���ƽ���ɣ��ʴ�Ϊ��ˮ����ǣ�������ð����CaCl2+Na2CO3=CaCO3��+2NaCl

��ϰ��ϵ�д�
�����Ŀ
