��Ŀ����
����Ҫ��������и���
��1��д������Ԫ�صķ��Ż����ƣ�
��2��д���������ʵ����ƻ�ѧʽ��
�Ȼ���
��3���û�ѧ������գ�
4��������̼����
��1��д������Ԫ�صķ��Ż����ƣ�
| Ԫ�ط��� | S S |
Ca | Hg Hg |
Au |
| Ԫ������ | �� | �� �� |
�� | �� �� |
�Ȼ���
NaCl
NaCl
��ˮH2O
H2O
���Ȼ���BaCl2
BaCl2
������
����
N2������������
����������
P2O5����������
��������
FeSO4����3���û�ѧ������գ�
4��������̼����
4CO2
4CO2
��2����ԭ��2K
2K
��8��������8Al3+
8Al3+
��1�������H2
H2
����������1������Ԫ�ط��ŵ���дԭ��һ���С��������һ����ĸ��д���ڶ�����ĸСд����д����Ԫ�ص�Ԫ�ط��ţ����Ծݴ��������⣻
��2���ɻ���������ƿ���֪�����ʵ�Ԫ����ɣ����ݻ�����Ļ�ѧʽ��д���ͻ��ϼ۾���д��������Ļ�ѧʽ��ֻ�����Ԫ�ط��ź�Ԫ�����ƣ����ܸ��ݻ�ѧʽ�Ķ����õ����ʵ����ƣ�
��3�����ݷ����ø����ʵĻ�ѧʽ��ʾ����������ڻ�ѧʽǰ�����֣�
����ԭ�ӵı�ʾ��������Ԫ�ط�������ʾһ��ԭ�ӣ���ʾ�����ԭ�ӣ�������Ԫ�ط���ǰ������Ӧ�����ֽ��н��
�������ӵı�ʾ�������ڱ�ʾ�����ӵ�Ԫ�ط������Ͻǣ���������������������������������ǰ�����������ں�1�����ʱ��1Ҫʡ�ԣ�����ʾ��������ӣ�������Ԫ�ط���ǰ������Ӧ�����֣����н��
��2���ɻ���������ƿ���֪�����ʵ�Ԫ����ɣ����ݻ�����Ļ�ѧʽ��д���ͻ��ϼ۾���д��������Ļ�ѧʽ��ֻ�����Ԫ�ط��ź�Ԫ�����ƣ����ܸ��ݻ�ѧʽ�Ķ����õ����ʵ����ƣ�
��3�����ݷ����ø����ʵĻ�ѧʽ��ʾ����������ڻ�ѧʽǰ�����֣�
����ԭ�ӵı�ʾ��������Ԫ�ط�������ʾһ��ԭ�ӣ���ʾ�����ԭ�ӣ�������Ԫ�ط���ǰ������Ӧ�����ֽ��н��
�������ӵı�ʾ�������ڱ�ʾ�����ӵ�Ԫ�ط������Ͻǣ���������������������������������ǰ�����������ں�1�����ʱ��1Ҫʡ�ԣ�����ʾ��������ӣ�������Ԫ�ط���ǰ������Ӧ�����֣����н��
����⣺��1�����Ԫ�ط���ΪS��CaΪ��Ԫ�ص�Ԫ�ط��ţ�����Ԫ�ط���ΪHg��AuΪ���Ԫ�ط��ţ�
��2���Ȼ����������Ӻ������ӹ��ɵģ�������Ԫ�صĻ��ϼ�Ϊ+1������Ԫ�صĻ��ϼ�Ϊ-1�����ݻ��������������ϵĴ�����Ϊ�����֪���Ȼ��ƵĻ�ѧʽΪ��NaCl��
ˮ����Ԫ�غ���Ԫ����ɣ�������Ԫ�صĻ��ϼ�Ϊ+1������Ԫ�صĻ��ϼ�Ϊ-1�����ݻ��������������ϵĴ�����Ϊ�����֪��ˮ�Ļ�ѧʽΪ��H2O��
�Ȼ�������Ԫ�غͱ�Ԫ����ɣ����б�Ԫ�صĻ��ϼ�Ϊ+2������Ԫ�صĻ��ϼ�Ϊ-1�����ݻ��������������ϵĴ�����Ϊ�����֪���Ȼ����Ļ�ѧʽΪ��BaCl2��
N2Ϊ�����Ļ�ѧʽ��P2O5Ϊ���������Ļ�ѧʽ��FeSO4������Ϊ����������
��3�����ݷ����ø����ʵĻ�ѧʽ��ʾ����������ڻ�ѧʽǰ�����֣�����4��������̼���ӿ��Ա�ʾΪ��4CO2��
����ԭ�ӵı�ʾ��������Ԫ�ط�������ʾһ��ԭ�ӣ���ʾ�����ԭ�ӣ�������Ԫ�ط���ǰ������Ӧ�����֣�����2����ԭ�ӿ��Ա�ʾΪ��2K��
�������ӵı�ʾ�������ڱ�ʾ�����ӵ�Ԫ�ط������Ͻǣ���������������������������������ǰ�����������ں�1�����ʱ��1Ҫʡ�ԣ�����ʾ��������ӣ�������Ԫ�ط���ǰ������Ӧ�����֣�����8�������ӿ��Ա�ʾΪ��8Al3+��
�������ʵĻ�ѧʽ���Ա�ʾ�����ʵ�һ�����ӣ�����1������ӿ��Ա�ʾΪ��H2��
�ʴ�Ϊ����1��
��2��NaCl��H2O��BaCl2�����������������ף�����������
��3��4CO2��2K��8Al3+��H2��
��2���Ȼ����������Ӻ������ӹ��ɵģ�������Ԫ�صĻ��ϼ�Ϊ+1������Ԫ�صĻ��ϼ�Ϊ-1�����ݻ��������������ϵĴ�����Ϊ�����֪���Ȼ��ƵĻ�ѧʽΪ��NaCl��
ˮ����Ԫ�غ���Ԫ����ɣ�������Ԫ�صĻ��ϼ�Ϊ+1������Ԫ�صĻ��ϼ�Ϊ-1�����ݻ��������������ϵĴ�����Ϊ�����֪��ˮ�Ļ�ѧʽΪ��H2O��
�Ȼ�������Ԫ�غͱ�Ԫ����ɣ����б�Ԫ�صĻ��ϼ�Ϊ+2������Ԫ�صĻ��ϼ�Ϊ-1�����ݻ��������������ϵĴ�����Ϊ�����֪���Ȼ����Ļ�ѧʽΪ��BaCl2��
N2Ϊ�����Ļ�ѧʽ��P2O5Ϊ���������Ļ�ѧʽ��FeSO4������Ϊ����������
��3�����ݷ����ø����ʵĻ�ѧʽ��ʾ����������ڻ�ѧʽǰ�����֣�����4��������̼���ӿ��Ա�ʾΪ��4CO2��
����ԭ�ӵı�ʾ��������Ԫ�ط�������ʾһ��ԭ�ӣ���ʾ�����ԭ�ӣ�������Ԫ�ط���ǰ������Ӧ�����֣�����2����ԭ�ӿ��Ա�ʾΪ��2K��
�������ӵı�ʾ�������ڱ�ʾ�����ӵ�Ԫ�ط������Ͻǣ���������������������������������ǰ�����������ں�1�����ʱ��1Ҫʡ�ԣ�����ʾ��������ӣ�������Ԫ�ط���ǰ������Ӧ�����֣�����8�������ӿ��Ա�ʾΪ��8Al3+��
�������ʵĻ�ѧʽ���Ա�ʾ�����ʵ�һ�����ӣ�����1������ӿ��Ա�ʾΪ��H2��
�ʴ�Ϊ����1��
| Ԫ�ط��� | S | Ca | Hg | Au |
| Ԫ������ | �� | �� | �� | �� |
��3��4CO2��2K��8Al3+��H2��
������������Ҫ����ѧ���Ի�ѧ�������д��������������Ŀ��ƼȰ����Ի�ѧ����������˽⣬�ֿ�����ѧ���Ի�ѧ���ŵ���д������ȫ�棬ע�ػ�����

��ϰ��ϵ�д�
�����Ŀ
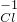 ______����CO3______�����������-3�۵ĵ�Ԫ��______��
______����CO3______�����������-3�۵ĵ�Ԫ��______�� ����CO3 �����������-3�۵ĵ�Ԫ�� ��
����CO3 �����������-3�۵ĵ�Ԫ�� ��