��Ŀ����
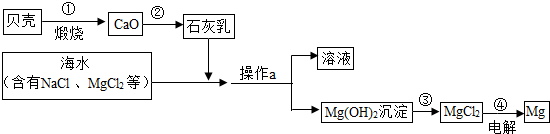
��ˮ���д����������õĻ�ѧ��Դ�������Ȼ�þ���Ȼ��ơ��廯�صȣ��ۺ����ú�ˮ�Ʊ�����þ��������ͼ��ʾ��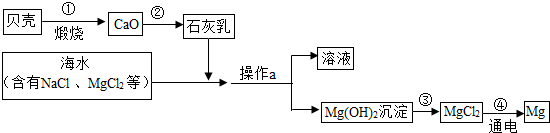
��1��������Ҫ�ɷֵĻ�ѧʽ��
��2������a��������
��3����ҵұ��þ���õ��MgCl2�ķ�������ӦΪ��MgCl2
| ||
��4��д���ڢڡ���������Ӧ�Ļ�ѧ����ʽ��
��
��������1�����ݱ��ǵ���Ҫ�ɷַ������ɣ�
��2�����ݹ��˲��������÷�Χ�����õ��������������ɣ�
��3������Ԫ���غ�������ɣ�
��4���ڸ�����������ˮ�ķ�Ӧԭ���������ɣ�
�۸���������þ������ķ�Ӧԭ���������ɣ�
��2�����ݹ��˲��������÷�Χ�����õ��������������ɣ�
��3������Ԫ���غ�������ɣ�
��4���ڸ�����������ˮ�ķ�Ӧԭ���������ɣ�
�۸���������þ������ķ�Ӧԭ���������ɣ�
����⣺��1�����ǵ���Ҫ�ɷ���̼��ƣ���ѡCaCO3
��2����Ϊ������þΪ������������ˮ�����Կ���ͨ�����˷��룬����ʱ��Ҫ�IJ����������ձ�����������©���ȣ�
�ʴ�Ϊ�����ˡ�©����
��3������Ԫ���غ��֪���ﻹ��������
��ѡCl2
��4����ʯ�������Ҫ�ɷ����������ƣ���������ˮ�ķ�Ӧ����ʽΪ��CaO+H2O=Ca��OH��2
��������þ�����ᷴӦ�ķ���ʽΪ��Mg��OH��2+2HCl=MgCl2+2H2O
��ѡCaO+H2O=Ca��OH��2��Mg��OH��2+2HCl=MgCl2+2H2O��
��2����Ϊ������þΪ������������ˮ�����Կ���ͨ�����˷��룬����ʱ��Ҫ�IJ����������ձ�����������©���ȣ�
�ʴ�Ϊ�����ˡ�©����
��3������Ԫ���غ��֪���ﻹ��������
��ѡCl2
��4����ʯ�������Ҫ�ɷ����������ƣ���������ˮ�ķ�Ӧ����ʽΪ��CaO+H2O=Ca��OH��2
��������þ�����ᷴӦ�ķ���ʽΪ��Mg��OH��2+2HCl=MgCl2+2H2O
��ѡCaO+H2O=Ca��OH��2��Mg��OH��2+2HCl=MgCl2+2H2O��
���������⿼���˴Ӻ�ˮ����ȡþ��ԭ���ͷ��������Ȼ�þ����ȡþ���ʣ�Ӧ�������̬���Ȼ�þ�����������û��ķ����õ�þ��

��ϰ��ϵ�д�
�����Ŀ