��Ŀ����
̼�ڵؿ��еĺ������ߣ������Ļ����������ڶ࣬���ҷֲ�����,̼��̼�Ļ���������������������Ӧ�ù㷺��
������ͼ����ѧ֪ʶ�ش��������⣺

��1����ͼ�����ڽ��ʯ�ṹ���� ����дͼ��ţ���ͼ��Ҳ��̼��һ�ֵ��ʣ��仯ѧʽΪ ��
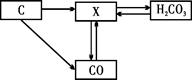
��2��X��Y��Z�dz��л�ѧ���������ʣ�����֮������ͼ��ʾ��ת����ϵ��X���ж����壬Y�Dz�֧��ȼ�յ����壬��ش��������⡣

��X�Ļ�ѧʽΪ___________��
��Y��ʯ��ˮ��Ӧ����Z�Ļ�ѧ����ʽΪ____ _____��
������X��Y������Ԫ����ͬ�������ǵĻ�ѧ���ʲ�ͬ����ԭ����_______ ____��
��3����̼��������ͭ��Ϻ��ǿ�ȣ�������Ӧ�Ļ�ѧ����ʽΪ_____ __ _____��
��4��CO2�ǿ������õ���Ҫ��Դ�������˵����������ש���𣿾�����һ�������£�CO2�ͽ����Ʒ�Ӧ������ȡ���ʯ����Ӧ�ķ���ʽ��CO2+4Na C(���ʯ)+2 X ,���Ƴ�X�Ļ�ѧʽ�� ��
C(���ʯ)+2 X ,���Ƴ�X�Ļ�ѧʽ�� ��
������ͼ����ѧ֪ʶ�ش��������⣺

��1����ͼ�����ڽ��ʯ�ṹ���� ����дͼ��ţ���ͼ��Ҳ��̼��һ�ֵ��ʣ��仯ѧʽΪ ��
��2��X��Y��Z�dz��л�ѧ���������ʣ�����֮������ͼ��ʾ��ת����ϵ��X���ж����壬Y�Dz�֧��ȼ�յ����壬��ش��������⡣

��X�Ļ�ѧʽΪ___________��
��Y��ʯ��ˮ��Ӧ����Z�Ļ�ѧ����ʽΪ____ _____��
������X��Y������Ԫ����ͬ�������ǵĻ�ѧ���ʲ�ͬ����ԭ����_______ ____��
��3����̼��������ͭ��Ϻ��ǿ�ȣ�������Ӧ�Ļ�ѧ����ʽΪ_____ __ _____��
��4��CO2�ǿ������õ���Ҫ��Դ�������˵����������ש���𣿾�����һ�������£�CO2�ͽ����Ʒ�Ӧ������ȡ���ʯ����Ӧ�ķ���ʽ��CO2+4Na
 C(���ʯ)+2 X ,���Ƴ�X�Ļ�ѧʽ�� ��
C(���ʯ)+2 X ,���Ƴ�X�Ļ�ѧʽ�� ����1��ͼ�� ��C60 (2) CO ��CO2 + Ca(OH)2��CaCO3��+ H2O �����ɵķ��Ӳ�ͬ
��3��C + CuO Cu + CO2 (4) Na2O
Cu + CO2 (4) Na2O
��3��C + CuO
 Cu + CO2 (4) Na2O
Cu + CO2 (4) Na2O ��������� ���ʯ��Ӳ����Ϊ������������ṹ��ѡͼ�ڣ�ͼ�ܻ�ѧʽC60����ͼ֪X��C��Y��CO2,Z��CaCO3, Y��ʯ��ˮ��Ӧ����Z�Ļ�ѧ����ʽΪCO2 + Ca(OH)2��CaCO3��+ H2O,CO��CO2��ѧ���ʲ�ͬԭ���ǹ��ɵķ��Ӳ�ͬ����̼��������ͭ��Ϻ��ǿ�ȣ�������Ӧ�Ļ�ѧ����ʽΪC + CuO
 Cu + CO2����Ӧ�ķ���ʽ��CO2+4Na
Cu + CO2����Ӧ�ķ���ʽ��CO2+4Na C(���ʯ)+2 X ,���������غ㶨�ɣ��Ƴ�X��ѧʽΪNa2O��
C(���ʯ)+2 X ,���������غ㶨�ɣ��Ƴ�X��ѧʽΪNa2O��
��ϰ��ϵ�д�
�����Ŀ


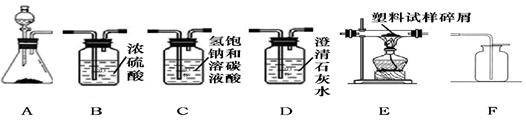
 2Cu + X��������̼�Ļ�ԭ��
2Cu + X��������̼�Ļ�ԭ��