��Ŀ����
ˮ��һ����Ҫ����Ȼ��Դ�������������������������أ�
��1����������Ȼ����ˮѭ����һ�����ڣ����÷����˶��Ĺ۵������һ�仯���� ��
��2��pH��5.6�Ľ�ˮ��Ϊ���꣬Ҫ����ij�ν�ˮ�Ƿ������꣬��ȷ�IJ���Ϊ ��
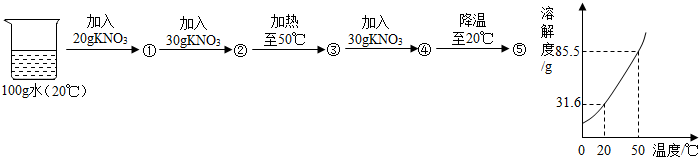
��3��ˮ�dz��õ��ܼ�����20mLˮ���ܽ�һ����KNO3����IJ������£������ʵ��������ܽ�����ش��������⣨ˮ���ܶ���1g/mL�ƣ���

A��B��C����Һһ���DZ�����Һ���� ��һ���Dz�������Һ���� ����X= gʱ��B��ǡ��û�й���ʣ�࣮
��1��ˮ����ʧȥ������ˮ�����˶����ʼ��������Ӽ����С����ˮ�������Һ�壻��2���ڲ���Ƭ�ϻ�״ɰ��Ϸ�һƬpH��ֽ���ò�����պȡ��ˮ����pH��ֽ�ϣ�����ʾ����ɫ�����ɫ���Աȣ���pHС��5.6����Ϊ���꣮��pH����5.6���������꣮��3��B��A��1.32��
��������
�����������1��ˮ����ˮ���ӹ��ɵģ�ˮ�������¶Ƚ���ʱ��ˮ����ʧȥ������ˮ�����˶����ʼ��������Ӽ����С���Ӷ�ˮ����������Һ����γ��˽��ꣻ
��2�����齵ˮ�Ƿ������꣬Ӧʹ��pH��ֽ���ⶨʱ���ڲ���Ƭ�ϻ�״ɰ��Ϸ�һƬpH��ֽ���ò�����պȡ��ˮ����pH��ֽ�ϣ�����ʾ����ɫ�����ɫ���Աȣ���pHС��5.6����Ϊ���꣮��pH����5.6���������ꣻ
��3����B��Һ�У���δ�ܽ��KNO3���壬һ����KNO3�ı�����Һ�����ܽ�����߿�֪����20��Cʱ������ص��ܽ����31.6�ˣ����ܽ�ȵĺ����֪����20mLˮ��20gˮ�����ܽ�6.32g�����ԣ�A��Һһ���Dz�������Һ��������B��Һ�����ܽ���5g����أ���X=1.32gʱ��B��ǡ�ôﵽ����û�й���ʣ�࣮
���㣺���÷�����ԭ�ӵ����ʷ����ͽ�����⣻��Һ�����Ȳⶨ��������Һ�Ͳ�������Һ�������ܽ�����������ã�
������������Ҫ��������ˮ�йص�֪ʶ����Ϥˮ���۹��ɡ�����pHʵ�ʲⶨ��ˮ�����ȣ�ȷ���ⱥ����Һ����������Һ���ܽ�ȵĶ����ǽ����Ĺؼ���

 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
 ��2013?�������ģ��ˮ��һ����Ҫ����Ȼ��Դ����������������ز����ٵ����ʣ�
��2013?�������ģ��ˮ��һ����Ҫ����Ȼ��Դ����������������ز����ٵ����ʣ�