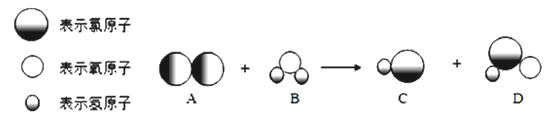
��Ŀ����
����Ŀ��̼��̼�Ļ����ﶼ�Ƿdz���Ҫ�����ʡ�

(1)̼�ĵ����ж��֣�A��B��C�У��ɷ��ӹ��ɵ���_______(�����)��
(2)�Ŵ���ī��д����Ƶ��ֻ�������Բ���ɫ��ԭ����ī����Ҫ�ɷ�̼�ڳ����¾���____________�ԣ�����̼ԭ�ӵĽṹʾ��ͼ________________����ľ̿��ԭ����ͭ��ʵ���У�������̼�Ļ�ԭ�ԣ��䷴Ӧ�Ļ�ѧ����ʽ��______________________________������̼�Ļ�ԭ�Կ�������ұ��ҵ��
(3)������̼��һ�ֱ������Դ���̶������ö�����̼��һ���ɹ������ǣ��ڸ��¸�ѹ��������CO2��NH3���Ժϳ�����[CO(NH2)2]��ͬʱ����ˮ���÷�Ӧ�Ļ�ѧ����ʽΪ_________________��
���𰸡�C�ȶ�![]() C��2CuO
C��2CuO![]() 2Cu��CO2��CO2��2NH3
2Cu��CO2��CO2��2NH3![]() CO(NH2)2��H2O
CO(NH2)2��H2O
��������
��1�����ʯ��̼ԭ��ֱ�ӹ��ɣ�ʯīҲ����̼ԭ��ֱ�ӹ��ɣ�C60��C60���ӹ��ɣ�����C��
��2��̼�ڳ����»�ѧ���ʱȽ��ȶ����ʹŴ���ī��д����Ƶ��ֻ�������Բ���ɫ��̼ԭ�ӵĽṹʾ��ͼ![]() ��ľ̿��ԭ����ͭ�Ļ�ѧ����ʽΪC��2CuO
��ľ̿��ԭ����ͭ�Ļ�ѧ����ʽΪC��2CuO![]() 2Cu��CO2����ע������Ϊ���£�������̼��������Ų�Ҫ��©��
2Cu��CO2����ע������Ϊ���£�������̼��������Ų�Ҫ��©��
��3���ڸ��¸�ѹ��������CO2��NH3���Ժϳ�����[CO(NH2)2]��ͬʱ����ˮ���÷�Ӧ�Ļ�ѧ����ʽ�ɱ�ʾΪCO2��2NH3![]() CO(NH2)2��H2O��ע�ⲻҪ������Ӧ��������ƽ��ѧ��������
CO(NH2)2��H2O��ע�ⲻҪ������Ӧ��������ƽ��ѧ��������

 �Ķ��쳵ϵ�д�
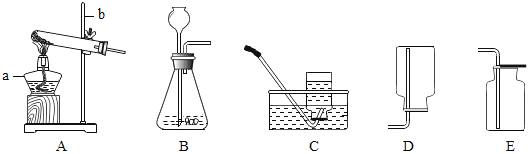
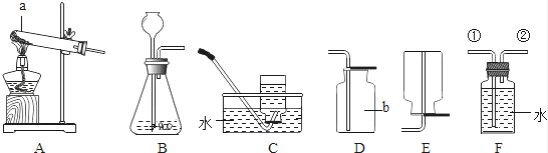
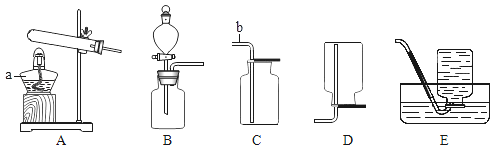
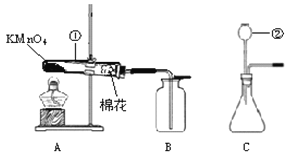
�Ķ��쳵ϵ�д�����Ŀ��ʵ���Ҳ���װ����ͼ��ʾ����ش��������⣮

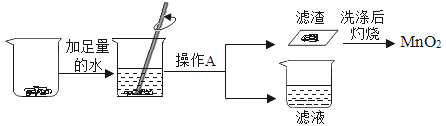
��1��ѡ�������ռ�O2��װ�ã���д���±��У�����ĸ����
ѡ��ҩƷ | ����װ�� | �ռ�װ�� |
H2O2��Һ��MnO2 | ______ | ______ |
KMnO4 | ______ |
��2�������MnO2��ŨH2SO4�����Ʊ�O2����ѡ�õķ���װ����______������ĸ����
��3��ѡ��Fװ���ռ�����ʱ������ʵ�������ȷ����______������ţ���
�ٷ�Ӧǰ��������ƿע��ˮ���ò���Ƭ����ƿ�ڣ�������ʢˮ��ˮ����
�ڿ�ʼ��Ӧ�ȵ����������Ҿ���ʱ���ٽ����ܿ����뼯��ƿ
���ռ����������ƿ���ϲ���Ƭ���Ƴ�ˮ��
��4��ʵ������KMnO4�Ʊ�O2�Ļ�ѧ����ʽ��______�������Ʊ�3.2g O2��������Ҫ����______gKMnO4����֪KMnO4����Է�������Ϊ158����