��Ŀ����
��ʡ�����з���һ��������Ĺ��������ѧ��ȷ��Ϊ�ܲٸ��꣬���н�����ͭ�����ȶ��ֽ����ʵص�����������ѧ֪ʶ�ش��������⣺
��1���ܲ�Ĺ���д�������ͭ�����������γ����ڣ����Ƿ��ֽ���������һ��Ȫ���������ֿ�Ȫˮ��õ�������CuSO4��5H2O��������Ȫˮ�������þ��˻��ͭ������Ҳ��ʪ����ͭ����Դ��д�������Ӧ�Ļ�ѧ����ʽ �������Ӧ�������� ��Ӧ�����õ�ͭ��Ʒ�������ʴ����ͭ��[Cu2(OH)2CO3]��ͭ����ͭ������е�������ˮ�� ��ͬ���õĽ����
��2���ܲ�Ĺ�е��������⼣�߰ߣ��������ǽ�һ����ű�ұ��ʹ�õģ����Ļ�Ա���ǿ������ȴ�ڿ����б��ֳ����õĿ���ʴ���ܣ���ԭ���� ��
��3���ִ������������У�����ʹ�õIJ��Ǵ��������������ǵĺϽ𡣺Ͻ�ʹ������Ƚϣ�����Խ�������� �����һ�����ɣ�
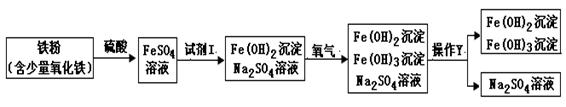
��4��(3��)Ϊ̽������ͭ�����Ļ��˳��ijͬѧ�����һ��ʵ�飺����Ƭ����ϡ������ ��ͭƬ����ϡ������ ����Ƭ����ϡ�����С� ����ʵ�黹������ȫ֤�����ֽ����Ļ��˳���벹��һ��ʵ�����ﵽĿ��(д��ʵ�鲽������ͻ�ѧ����ʽ)��
��5��(3��)��2000t��������80%�ij�����ʯ�������Ͽ�����������96%�������������Ƕ��٣�
��1���ܲ�Ĺ���д�������ͭ�����������γ����ڣ����Ƿ��ֽ���������һ��Ȫ���������ֿ�Ȫˮ��õ�������CuSO4��5H2O��������Ȫˮ�������þ��˻��ͭ������Ҳ��ʪ����ͭ����Դ��д�������Ӧ�Ļ�ѧ����ʽ �������Ӧ�������� ��Ӧ�����õ�ͭ��Ʒ�������ʴ����ͭ��[Cu2(OH)2CO3]��ͭ����ͭ������е�������ˮ�� ��ͬ���õĽ����
��2���ܲ�Ĺ�е��������⼣�߰ߣ��������ǽ�һ����ű�ұ��ʹ�õģ����Ļ�Ա���ǿ������ȴ�ڿ����б��ֳ����õĿ���ʴ���ܣ���ԭ���� ��
��3���ִ������������У�����ʹ�õIJ��Ǵ��������������ǵĺϽ𡣺Ͻ�ʹ������Ƚϣ�����Խ�������� �����һ�����ɣ�
��4��(3��)Ϊ̽������ͭ�����Ļ��˳��ijͬѧ�����һ��ʵ�飺����Ƭ����ϡ������ ��ͭƬ����ϡ������ ����Ƭ����ϡ�����С� ����ʵ�黹������ȫ֤�����ֽ����Ļ��˳���벹��һ��ʵ�����ﵽĿ��(д��ʵ�鲽������ͻ�ѧ����ʽ)��
��5��(3��)��2000t��������80%�ij�����ʯ�������Ͽ�����������96%�������������Ƕ��٣�
Fe+CuSO4=FeSO4+H2 �û� ������̼
���ı�����һ�����ܵ������ﱡĤ
�Ͻ��Ӳ�ȱȴ�������Ӳ��ǿ�����������𰸶��ɣ�
��3�֣���ͭƬ���뵽��������Һ�У�����ͭƬ����������ɫ���ʣ���Һ����ɫ�����ɫ
Cu+2AgNO3=" Cu" (NO3) 2+2Ag
(3��)1167t��
���ı�����һ�����ܵ������ﱡĤ
�Ͻ��Ӳ�ȱȴ�������Ӳ��ǿ�����������𰸶��ɣ�
��3�֣���ͭƬ���뵽��������Һ�У�����ͭƬ����������ɫ���ʣ���Һ����ɫ�����ɫ
Cu+2AgNO3=" Cu" (NO3) 2+2Ag
(3��)1167t��
��1�������Ϳ�Ȫˮ����Ҫ�ɷ�CuSO4�����û���Ӧ��Fe+CuSO4=FeSO4+H2������ͭ��������Ԫ���غ㣬ͭ��[Cu2(OH)2CO3] ��ͭ������е�������ˮ�ͺ�̼Ԫ�ص�CO2��Ӧ��
��2�����Ϳ����е�O2��Ӧ����Al2O3,�����γ�һ�����ܵ������ﱡĤ��
��3���Ͻ�ʹ������Ƚϣ�����Խ���������۵㡢�е�͡�ǿ�ȸߡ�Ӳ�ȴ����ѡһ����
��4��ʵ�����Ƭ����ϡ������ ��ͭƬ����ϡ������ ����Ƭ����ϡ�����У����ܱȽϳ�������������ڣ�ͭ��������Ҫ�Ƚ�ͭ�����Ľ�����Ի���Ҫ����ͭƬ����������Ӧʵ�顣
��5���⣺�������Ͽ�������������������X
Fe2O3 �� 2 Fe
160 112
2000t��80% X
160: 2000t��80% = 112:X
X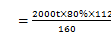 = 1120t
= 1120t
����96%��������������: 1120t��96%=1167t
�������Ͽ�����������96%��������������1167t.
��2�����Ϳ����е�O2��Ӧ����Al2O3,�����γ�һ�����ܵ������ﱡĤ��
��3���Ͻ�ʹ������Ƚϣ�����Խ���������۵㡢�е�͡�ǿ�ȸߡ�Ӳ�ȴ����ѡһ����
��4��ʵ�����Ƭ����ϡ������ ��ͭƬ����ϡ������ ����Ƭ����ϡ�����У����ܱȽϳ�������������ڣ�ͭ��������Ҫ�Ƚ�ͭ�����Ľ�����Ի���Ҫ����ͭƬ����������Ӧʵ�顣
��5���⣺�������Ͽ�������������������X
Fe2O3 �� 2 Fe
160 112
2000t��80% X
160: 2000t��80% = 112:X
X
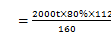 = 1120t
= 1120t����96%��������������: 1120t��96%=1167t
�������Ͽ�����������96%��������������1167t.

��ϰ��ϵ�д�
 �����Ļ�������ҵϵ�д�
�����Ļ�������ҵϵ�д�
�����Ŀ