��Ŀ����
����Ŀ����Դ�ͻ�����Ϊ���������ע�����⣮Ŀǰú̿���ҹ���Դ�ṹ��ռ�еı������
��1���ҹ������ƹ��ͥ������Ȼ������ú��ȼ�ϣ���Ȼ������Ҫ�ɷ��� ��
��2������úȼ�ջ����������Ⱦ�����ж��������ŷŵ������лᵼ�µĻ��������� ��
��3��Ϊ���θ���Ⱦ��ij����������µ����۷��������������˶��������õ���ij�ֻ�����Ʒ�� �ù���������ͼ��ʾ��
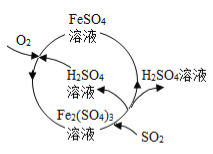
��Fe2��SO4��3��H2O����SO2����FeSO4��H2SO4�Ļ�ѧ����ʽ�� ��
�ڸù����пɵõ��Ļ�����Ʒ�� ��
��4��ΪӦ�����Ƕ���Դ�ͻ�����������Ҫ����Լ������Դ����������Դ�������뷢չ�����������������ϸ�������� ������ţ���
���ݶ���װ̫���ܷ���װ��
�ڷ������ոѣ���ľ�ҷ���
���Ż�������ƣ����ٿյ�ʹ��
�ܳ��������ͨ������˽�ҳ�ʹ�ã�
���𰸡�
��1�����飻
��2�����ꣻ
��3����Fe2��SO4��3+2H2O+SO2=2FeSO4+2H2SO4�����������������
��4���٢ۢ�
��������
���������
��1����Ȼ������Ҫ�ɷ��Ǽ��飬������飻
��2�������������ˮ��Ӧ���������ᣬ�Ӷ��γ����꣬������ꣻ
��3����Fe2��SO4��3��H2O����SO2����FeSO4��H2SO4���÷�Ӧ�Ļ�ѧ����ʽΪFe2��SO4��3+2H2O+SO2=2FeSO4+2H2SO4�����Fe2��SO4��3+2H2O+SO2=2FeSO4+2H2SO4���ڸù����еõ��Ļ�����Ʒ���������������ᣬ����������������
��4�����ݶ���װ̫���ܷ���װ���ܽ�Լ��Դ����ȷ���ڷ������ոѣ���ľ�ҷ������ɻ�����Ⱦ�������Ż�������ƣ����ٿյ�ʹ�ÿ��Խ�Լ��Դ����ȷ���ܳ��������ͨ������˽�ҳ�ʹ�ÿ��Խ�Լ��Դ������������ȷ������٢ۢ�

 ״Ԫ����ϵ�д�
״Ԫ����ϵ�д� ͬ������ϵ�д�
ͬ������ϵ�д�����Ŀ����ȥ���������е��������ʣ���ѡ�õ��Լ��Ͳ�����������ȷ����
ѡ�� | ���� | ���� | �Լ� | �������� |
A | MnO2 | KCl | ����ˮ | �ܽ⡢���ˡ��������ᾧ |
B | CO2 | H2 | �������� | ��ȼ |
C | NaOH��Һ | Ca��OH��2 | ����Na2CO3��Һ | ���� |
D | ϡ���� | ���� | ������������Һ | ���� |