��Ŀ����
Ϊ�˶Ժ�ˮ�е��Ȼ��ƣ�������NaCl���㣩���гɷַ������ס��ҡ���������λͬѧ�ֱ����ʵ�飬���ǵ�ʵ���������±�������ϸ�۲�������ش��������⣺
| �� | �� | �� | �� | |
| ��ȡ��ˮ��Ʒ������/g | 100 | 100 | 100 | 300 |
| ����AgNO3��Һ������/g | 50 | 100 | 150 | 200 |
| ��Ӧ�����õij����������/g | 1.435 | 2.87 | 2.87 | 5.74 |
��2������㺣ˮ�е��Ȼ�����Ȼ��Ƽ��㣩�����������Ƕ��٣�
�⣺��1�������ݷֲ����ɿ�������2.87g������������Һ����100g���������Һû�з�Ӧ��
��1���ң�2�֣�
��2����NaCl������ΪX
NaCl+AgNO3=AgCl��+NaNO3
58.5 143.5
X 2.87g
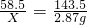
X=1.17g
ˮ�е��Ȼ�����Ȼ��Ƽ��㣩������������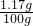 100%=1.17%
100%=1.17%
�𣺷�Ӧ��������������Һ��������������Ϊ1.17%��
�ʴ�Ϊ����1���ң���2��1.17%��
����������ʵ�����ݱ�����ȡ��ˮ��Ʒ�����������������Һ�����Ĺ�ϵ���ж�������Һǡ����ȫ��Ӧ��ʵ�飻�������ɳ����Ȼ������������ݷ�Ӧ�Ļ�ѧ����ʽ�����㺣ˮ���Ȼ��Ƶ���������������������������ļ��㣮
�������Ĵ�ʵ���к�ˮ��Ʒ������������������Һ�����ı仯�DZ�������Ĺؼ����Ա��Ĵ�ʵ����������Һ�����ɳ����Ĺ�ϵ�����ҳ�����Һ���з�Ӧ��������ϵ��
��1���ң�2�֣�
��2����NaCl������ΪX
NaCl+AgNO3=AgCl��+NaNO3
58.5 143.5
X 2.87g
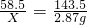
X=1.17g
ˮ�е��Ȼ�����Ȼ��Ƽ��㣩������������
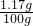 100%=1.17%
100%=1.17% �𣺷�Ӧ��������������Һ��������������Ϊ1.17%��
�ʴ�Ϊ����1���ң���2��1.17%��
����������ʵ�����ݱ�����ȡ��ˮ��Ʒ�����������������Һ�����Ĺ�ϵ���ж�������Һǡ����ȫ��Ӧ��ʵ�飻�������ɳ����Ȼ������������ݷ�Ӧ�Ļ�ѧ����ʽ�����㺣ˮ���Ȼ��Ƶ���������������������������ļ��㣮
�������Ĵ�ʵ���к�ˮ��Ʒ������������������Һ�����ı仯�DZ�������Ĺؼ����Ա��Ĵ�ʵ����������Һ�����ɳ����Ĺ�ϵ�����ҳ�����Һ���з�Ӧ��������ϵ��

��ϰ��ϵ�д�
 ��ĩ100�ִ��غ�������ϵ�д�
��ĩ100�ִ��غ�������ϵ�д� Сѧ�������Ծ�ϵ�д�
Сѧ�������Ծ�ϵ�д�
�����Ŀ
Ϊ�˶Ժ�ˮ�е��Ȼ��ƣ�������NaCl���㣩���гɷַ������ס��ҡ���������λͬѧ�ֱ����ʵ�飬���ǵ�ʵ���������±�������ϸ�۲�������ش��������⣺
| �� | �� | �� | �� | |
| ��ȡ��ˮ��Ʒ������/g | 100 | 100 | 100 | 300 |
| ����AgNO3��Һ������/g | 50 | 100 | 150 | 200 |
| ��Ӧ�����õij����������/g | 1.435 | 2.87 | 2.87 | 5.74 |
(1)����Һǡ����ȫ��Ӧ����________________����ס��ҡ�������ʵ�顣
(2)����㺣ˮ�е��Ȼ�����Ȼ��Ƽ��㣩�����������Ƕ��١�
Ϊ�˶Ժ�ˮ�е��Ȼ��ƣ�������NaCl���㣩���гɷַ������ס��ҡ���������λͬѧ�ֱ����ʵ�飬���ǵ�ʵ���������±�������ϸ�۲�������ش��������⣺
��1������Һǡ����ȫ��Ӧ����______����ס��ҡ�������ʵ�飮
��2������㺣ˮ�е��Ȼ�����Ȼ��Ƽ��㣩�����������Ƕ��٣�
| �� | �� | �� | �� | |
| ��ȡ��ˮ��Ʒ������/g | 100 | 100 | 100 | 300 |
| ����AgNO3��Һ������/g | 50 | 100 | 150 | 200 |
| ��Ӧ�����õij����������/g | 1.435 | 2.87 | 2.87 | 5.74 |
��2������㺣ˮ�е��Ȼ�����Ȼ��Ƽ��㣩�����������Ƕ��٣�