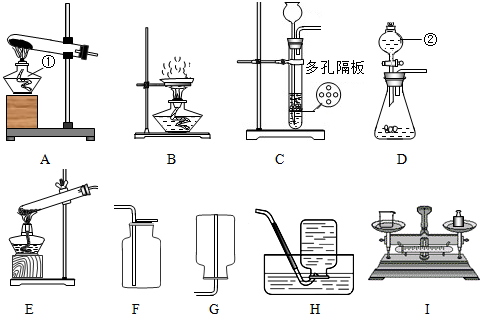
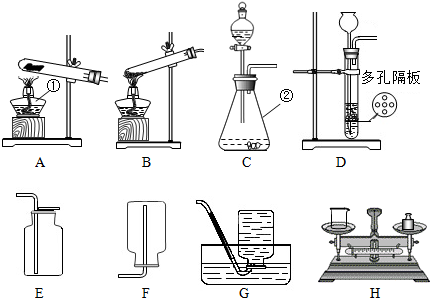
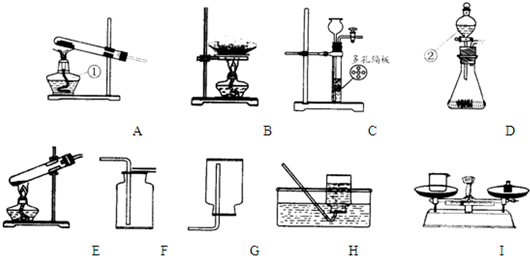
��Ŀ����
��������װ��ͼ��д�йؿո�
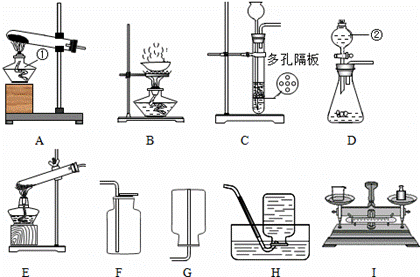
��1��װ��A�������ٵ�����Ϊ ______װ��D�������ڵ�����Ϊ ______
��2����ϡ�����п���������ķ���װ�ÿ�ѡ������______�� ______������ţ�װ�ã��ռ���������______�� ______������ţ�װ�ã�
��3����ȥ�����в��������ʵ�ʵ�鲽���� ___________________��
���˺������ᾧ���õ�������װ����______������ţ��������������в�����������е� ______������ţ��������ʵ�飮
��©�����ձ��������� �ڵιܡ���Ͳ���Թ� �ۼ���ƿ���������ƿ
��4�����Ȼ��ƹ����ˮ����5%���Ȼ�����Һ����Ҫ�õ���ͼװ���е�______������ţ���
�����ƹ����У����Ѿ�������ˮ���ձ��м���5g�Ȼ��ƺ�95mLˮ�������������淶����������Һ���Ȼ��Ƶ��������� ______���������������=����5%��
��1���ƾ��ƣ���Һ©��
��2��C��D��G��H����
��3���ܽ⣬B�� ��
��4��I������

��ϰ��ϵ�д�
�����Ŀ