��Ŀ����
��ʡij���п���ѧʵ������������ĸ����⣺������һ������������������Һ���ڼ�Ļ�ѧ���ʣ���CO2����ȡ���ռ�����������O2����ȡ���ռ������������Եķ������ɿ�����ǩȷ�����⣬С��ͬѧ��ǩ���ʦ��������������������ҩƷ��ʵ��̨ǰ����ش�
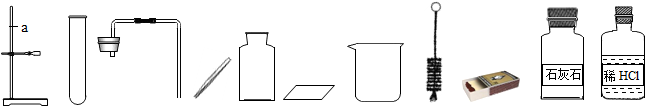
��1��ָ��ͼ1�б�����������ƣ�a______b______��
��2����ʵ��̨���ṩ��������ҩƷ������ΪС��鵽���ǵ�______�����⣻
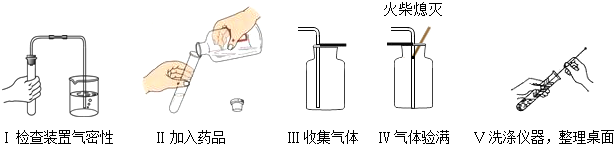
��3��ͼ2��С����ɸ�ʵ����Ҫ�������̵�ʾ��ͼ�������ֱ���ÿ�������ȷ��1�֣��ܹ�5�֣�ʵ����Ϻ�С�����3
�֣����ҳ�С��ʧ�ֵIJ�����˵��ԭ��______��______��

��4����������������ҩƷ��ѡ����Ҳ�������һ�ֳ��������ʵ����ȡ����ѧ����ʽΪ______��������______����һ�ֲ����������ƣ�������װ���������ȡ�����ķ���װ�ã�
���𰸡���������ȡװ�ð������ȺͲ���������֣������˫��ˮ�������Ͳ���Ҫ���ȣ�����ø�����ػ����������������Ҫ���ȣ��������ܶȱȿ������ܶȴ�������ˮ��������������ſ���������ˮ���ռ�����ʵ��̨���ṩ��������ҩƷ������ΪС��鵽����O2����ȡ���ռ���������С����ɸ�ʵ����Ҫ���������д�����У�����ֱ���Թܿڼ���ҩƷ��ҩƷ��մ�ڹܿڻ�ܱڣ��ô�����ľ������ƿ��������������֤�Ƿ��ռ�����ʵ������ȡCO2�����ڳ����£���̼��ƺ����ụ�ཻ���ɷ������Ȼ��ƺ�ˮ�Ͷ�����̼����˲���Ҫ���ȣ�������̼������ˮ���ܶȱȿ������ܶȴ����ֻ���������ſ������ռ���
����⣺��1���Թ��dz��õķ�Ӧ�������Թ�ˢ��ˢ�Թܵ��������ʴ�Ϊ��a���Թ�b���Թ�ˢ
��2����ʵ��̨���ṩ��������ҩƷ������ΪС��鵽����O2����ȡ���ռ����������ʴ�Ϊ����
��3��С����ɸ�ʵ����Ҫ���������д�����У�����ֱ���Թܿڼ���ҩƷ��ҩƷ��մ�ڹܿڻ�ܱڣ��ô�����ľ������ƿ��������������֤�Ƿ��ռ������ʴ�Ϊ������ֱ���Թܿڼ���ҩƷ��ҩƷ��մ�ڹܿڻ�ܱڣ��ô�����ľ������ƿ��������������֤�Ƿ��ռ���
��4��̼��ƺ����ụ�ཻ���ɷ������Ȼ��ƺ�ˮ�Ͷ�����̼����ƽ���ɣ������Ӿƾ��ƣ�������װ���������ȡ�����ķ���װ�ã��ʴ�Ϊ��CaCO3+2HCl=CaCl2+CO2��+H2O���ƾ���
��������������Ҫ���������������ơ��������ȡװ�ú��ռ�װ�õ�ѡ��ͬʱҲ�����˻�ѧ����ʽ����д��������������ۺ��ԱȽ�ǿ���������ȡװ�õ�ѡ���뷴Ӧ���״̬�ͷ�Ӧ�������йأ�������ռ�װ�õ�ѡ����������ܶȺ��ܽ����йأ����������п�����Ҫ����֮һ����Ҫ������ʵ�����У�
����⣺��1���Թ��dz��õķ�Ӧ�������Թ�ˢ��ˢ�Թܵ��������ʴ�Ϊ��a���Թ�b���Թ�ˢ
��2����ʵ��̨���ṩ��������ҩƷ������ΪС��鵽����O2����ȡ���ռ����������ʴ�Ϊ����
��3��С����ɸ�ʵ����Ҫ���������д�����У�����ֱ���Թܿڼ���ҩƷ��ҩƷ��մ�ڹܿڻ�ܱڣ��ô�����ľ������ƿ��������������֤�Ƿ��ռ������ʴ�Ϊ������ֱ���Թܿڼ���ҩƷ��ҩƷ��մ�ڹܿڻ�ܱڣ��ô�����ľ������ƿ��������������֤�Ƿ��ռ���
��4��̼��ƺ����ụ�ཻ���ɷ������Ȼ��ƺ�ˮ�Ͷ�����̼����ƽ���ɣ������Ӿƾ��ƣ�������װ���������ȡ�����ķ���װ�ã��ʴ�Ϊ��CaCO3+2HCl=CaCl2+CO2��+H2O���ƾ���
��������������Ҫ���������������ơ��������ȡװ�ú��ռ�װ�õ�ѡ��ͬʱҲ�����˻�ѧ����ʽ����д��������������ۺ��ԱȽ�ǿ���������ȡװ�õ�ѡ���뷴Ӧ���״̬�ͷ�Ӧ�������йأ�������ռ�װ�õ�ѡ����������ܶȺ��ܽ����йأ����������п�����Ҫ����֮һ����Ҫ������ʵ�����У�

��ϰ��ϵ�д�
�����Ŀ
��ʡij���п���ѧʵ������������ĸ����⣺�ٴ����ᴿ����Ļ�ѧ���ʢ۶�����̼����ȡ���ռ�����������������ȡ���ռ������������Եķ������ɿ�����ǩȷ�����⣬С��ͬѧ��ǩ���ʦ��������������������ҩƷ��ʵ��̨ǰ��

��ش�
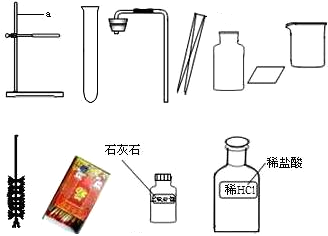
��ָ����ͼ������a������ ��
����ʵ��̨���ṩ��������ҩƷ������ΪС���鵽���ǵ� �����⣻
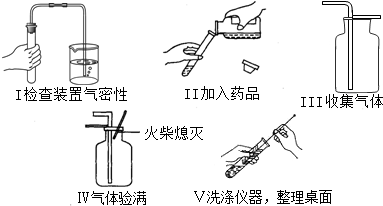
��������С����ɸ�ʵ����Ҫ�������̵�ʾ��ͼ�������ֱ���ÿ�������ȷ��1�֣�����5�֣�ʵ����Ϻ�С������3�֡����ҳ���ʧ�ֵIJ�����˵��ԭ��
�� ��
 |
�Ƚ�������������ҩƷ��ѡ����Ҳ�������һ�ֳ��������ʵ������ȡ����ѧ����ʽΪ�� ��