��Ŀ����
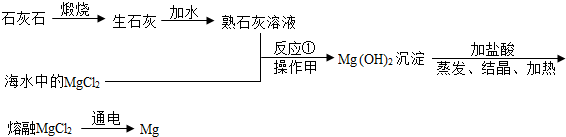
�����б��ٵı�������һ�������Դ�⣮���磬���ԴӺ�ˮ����ȡʳ�Σ�Ҳ���ԴӺ�ˮ����ȡ��;�㷺�Ľ���þ������ȡ�����������£�
��1���Ӻ�ˮ����ȡʳ�Σ�������______�ͷ���ʹˮ���������õ�ʳ�Σ�
��2����Ӧ�ٵĻ�ѧ����ʽ��______��
��3��������������______��
��4��С��۲쵽��ʦÿ����þ������ʵ��ʱ��þ������������һ��Һ�ɫ�ġ����¡�����ɰ����ȥ��¶������ɫ�Ľ��������������ʦ��֪������㡰���¡���þ���������ijЩ���ʻ������ɵ�һ�ֻ���������룬������MgO��Ҳ������Mg��OH��2����ʦ�������������á����¡���������MgO��ԭ����______
��5��С���ռ���һЩ����ĸá����¡�������һ�����������������������������м��ȣ��������ɵ���������ͨ��װ�и������ֻ����ˮ�֣��������µ����壩�ĸ���ܺ�װ����������ʯ��ˮ���ձ��У������������������ӣ�����ʯ��ˮ���ɫ���ǣ�
�ٸá����¡���������Mg��OH��2��ԭ���ǣ�����ֻ��ʵ������______��
����ɸá����¡�������Ԫ�ص�Ԫ����������______��
�۲μӷ�Ӧ���ɸá����¡�������������______��

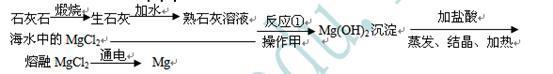
��1���Ӻ�ˮ����ȡʳ�Σ�������______�ͷ���ʹˮ���������õ�ʳ�Σ�
��2����Ӧ�ٵĻ�ѧ����ʽ��______��
��3��������������______��
��4��С��۲쵽��ʦÿ����þ������ʵ��ʱ��þ������������һ��Һ�ɫ�ġ����¡�����ɰ����ȥ��¶������ɫ�Ľ��������������ʦ��֪������㡰���¡���þ���������ijЩ���ʻ������ɵ�һ�ֻ���������룬������MgO��Ҳ������Mg��OH��2����ʦ�������������á����¡���������MgO��ԭ����______
��5��С���ռ���һЩ����ĸá����¡�������һ�����������������������������м��ȣ��������ɵ���������ͨ��װ�и������ֻ����ˮ�֣��������µ����壩�ĸ���ܺ�װ����������ʯ��ˮ���ձ��У������������������ӣ�����ʯ��ˮ���ɫ���ǣ�
�ٸá����¡���������Mg��OH��2��ԭ���ǣ�����ֻ��ʵ������______��
����ɸá����¡�������Ԫ�ص�Ԫ����������______��
�۲μӷ�Ӧ���ɸá����¡�������������______��
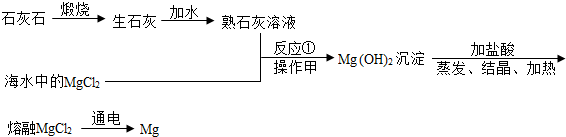
��1���Ӻ�ˮ����ȡʳ�Σ��������չ�ͷ���ʹˮ���������õ�ʳ�Σ�����չ⣮
��2���������ƺ��Ȼ�þ��Ӧ�Ļ�ѧ����ʽΪ��Ca��OH��2+MgCl2�TCaCl2+Mg��OH��2����
��3�������������ǹ��ˣ�������ˣ�
��4���á����¡���������MgO��ԭ���ǣ�MgO�ǰ�ɫ�����ˡ����¡��ǻҺ�ɫ�����MgO�ǰ�ɫ�����ˡ����¡��ǻҺ�ɫ��
��5���ٸá����¡���������Mg��OH��2��ԭ���ǣ�����ʯ��ˮ����ǣ�˵���ˡ����¡��ֽ����ɶ�����̼������̼Ԫ�أ���Mg��OH��2
�в���̼Ԫ�أ��������ʯ��ˮ����ǣ�˵���ˡ����¡��ֽ����ɶ�����̼������̼Ԫ�أ���Mg��OH��2
�в���̼Ԫ�أ�
����ɸá����¡�������Ԫ�ص�Ԫ�ط����ǣ�Mg��O��H��C�����Mg��O��H��C��
�۲μӷ�Ӧ���ɸá����¡������������ǣ�H2O��CO2��O2�����H2O��CO2��O2��
��2���������ƺ��Ȼ�þ��Ӧ�Ļ�ѧ����ʽΪ��Ca��OH��2+MgCl2�TCaCl2+Mg��OH��2����
��3�������������ǹ��ˣ�������ˣ�
��4���á����¡���������MgO��ԭ���ǣ�MgO�ǰ�ɫ�����ˡ����¡��ǻҺ�ɫ�����MgO�ǰ�ɫ�����ˡ����¡��ǻҺ�ɫ��
��5���ٸá����¡���������Mg��OH��2��ԭ���ǣ�����ʯ��ˮ����ǣ�˵���ˡ����¡��ֽ����ɶ�����̼������̼Ԫ�أ���Mg��OH��2
�в���̼Ԫ�أ��������ʯ��ˮ����ǣ�˵���ˡ����¡��ֽ����ɶ�����̼������̼Ԫ�أ���Mg��OH��2
�в���̼Ԫ�أ�
����ɸá����¡�������Ԫ�ص�Ԫ�ط����ǣ�Mg��O��H��C�����Mg��O��H��C��
�۲μӷ�Ӧ���ɸá����¡������������ǣ�H2O��CO2��O2�����H2O��CO2��O2��

��ϰ��ϵ�д�
�����Ŀ