��Ŀ����
����װ�ó�����ʵ������ȡ���壮���ݸ�����װ�ûش���������
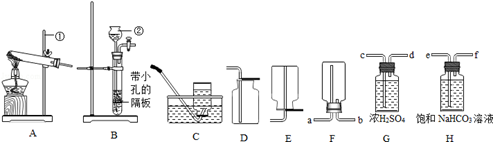
��1��ָ������������ƣ��� ��
��2��ʵ��������Aװ����ȡ��������Ӧԭ���û�ѧ����ʽ��ʾΪ ��
��3����ȡ���ռ�������̼Ӧѡ���װ���� ����A����E��ѡ��ʹ�ø���װ����ȡ�����ͻ���ŵ��� ���÷�Ӧԭ���û�ѧ����ʽ��ʾΪ ����Fװ���ռ�������̼��������Ӧ�� �˽��룮�ƵõĶ�����̼�г������������Ȼ���������ˮ��������ʹ��G��Hװ�ý��������������ȥ����װ����ȷ������˳���ǣ��������� ���ö˿���ĸ��ʾ����
��4���������Ķ�����̼����ͨ��ʢ������ˮ��ϴ��ƿһ��ʱ���ø�װ������Һ��pH�������������������=����7��

��1��ָ������������ƣ��� ��
��2��ʵ��������Aװ����ȡ��������Ӧԭ���û�ѧ����ʽ��ʾΪ ��
��3����ȡ���ռ�������̼Ӧѡ���װ���� ����A����E��ѡ��ʹ�ø���װ����ȡ�����ͻ���ŵ��� ���÷�Ӧԭ���û�ѧ����ʽ��ʾΪ ����Fװ���ռ�������̼��������Ӧ�� �˽��룮�ƵõĶ�����̼�г������������Ȼ���������ˮ��������ʹ��G��Hװ�ý��������������ȥ����װ����ȷ������˳���ǣ��������� ���ö˿���ĸ��ʾ����
��4���������Ķ�����̼����ͨ��ʢ������ˮ��ϴ��ƿһ��ʱ���ø�װ������Һ��pH�������������������=����7��
��1������©�� ��2��2KMnO4 K2MnO4+MnO2 +O2��
K2MnO4+MnO2 +O2��
��3��B��E ������ʱʹ��Ӧ���У�Ҳ������ʱʹ��Ӧֹͣ b e��f��c��d
��4����
 K2MnO4+MnO2 +O2��
K2MnO4+MnO2 +O2����3��B��E ������ʱʹ��Ӧ���У�Ҳ������ʱʹ��Ӧֹͣ b e��f��c��d
��4����
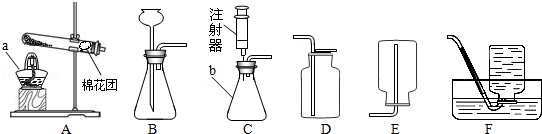
�����������1�����dz���©����
�������©����
��2��ʵ���ҿ����ü��ȸ�����صķ�����ȡ�����������������ʱ�ֽ�����������ء��������̺���������Ӧ�Ļ�ѧ����ʽΪ��2KMnO4
 K2MnO4+MnO2 +O2����
K2MnO4+MnO2 +O2�������2KMnO4
 K2MnO4+MnO2 +O2����
K2MnO4+MnO2 +O2������3����ȡ������̼������Bװ�ã�������̼���ܶȱȿ��������������ſ������ռ�����Eװ�ã�
���B��E��
ʹ�ø���װ����ȡ�����ͻ���ŵ��ǣ�������ʱʹ��Ӧ���У�Ҳ������ʱʹ��Ӧֹͣ��
���������ʱʹ��Ӧ���У�Ҳ������ʱʹ��Ӧֹͣ��
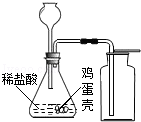
ʵ�����ô���ʯ��ʯ��ʯ��ϡ���ᷴӦ��ȡ������̼������ʯ��ʯ��ʯ����Ҫ�ɷ���̼��ƣ�̼��ƺ�ϡ���ᷴӦ�������Ȼ��ơ�ˮ�Ͷ�����̼����ѧ����ʽΪ��
CaCO3+2HCl=CaCl2+H2O+CO2����
���CaCO3+2HCl=CaCl2+H2O+CO2����
��Fװ���ռ�������̼ʱ������Ӧ��b�˽��룮
���b��
Ҫ��ȥ�Ȼ���������ˮ������Ӧ���ȳ�ȥ�Ȼ��⣬�ٳ�ȥˮ������˳��Ϊ����������e��f��c��d��
���e��f��c��d��
��4��������̼��ˮ��Ӧ����̼�ᣬ̼�������ԣ�pHС��7��
�������
������������Ҫ����ʵ����ơ�����ȥ���ͻ�ѧ����ʽ����д�ȷ����֪ʶ����д��ѧ����ʽʱҪע���IJ���һ�Ƿ�Ӧ���������Ļ�ѧʽҪ��ȷ��������ѭ�����غ㶨�ɣ�����д�ϱ�Ҫ�����������ǿ��Ƿ��С�����������

��ϰ��ϵ�д�
 �ִʾ�ƪ��ͬ�����Ĵ��ϵ�д�
�ִʾ�ƪ��ͬ�����Ĵ��ϵ�д�
�����Ŀ


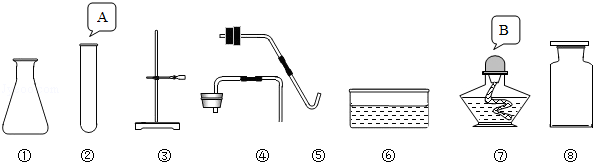

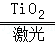 2H2��+O2��������˵��������ǣ�������
2H2��+O2��������˵��������ǣ�������