��Ŀ����
С��ͬѧ��ͨ��ʵ��̽��ij��ҵ���õİ���ƵĵĴ�����Ʒ�ijɷּ�������
��������⡿�ô�����Ʒ�к�����Щ���ʣ�
����������衿ͨ��������С���������¼��裺
��1��ֻ����Na2CO3 �� ��2������Na2CO3��NaHCO3
���������ϡ�
| NaHCO3 | Na2CO3 | |
| ����ϡ���� | ����� | �������� |
| ���뱥��ʯ��ˮ | ��Һ����� | ����� |
| ����CaCl2��Һ | ���������� | ��Һ����� |
| ������Һ�����ڣ�������ͨ�����ʯ��ˮ | ����ʯ��ˮ����� | ����ʯ��ˮ�����Ա仯 |
��1�����е������Ϊ ��
��2��������Ӧ�Ļ�ѧ����ʽΪ ��
��ʵ��̽����Ϊ��ȷ��������Ʒ�ijɷ֣�С���������ʵ�鷽��������һ�����С��ʵ�鱨�档
| ʵ�鲽�� | ʵ������ | |
| ��ȡ������Ʒ����ˮ�����������CaCl2��Һ�� | �� | �÷�Ӧ�ķ���ʽ�� �� |
| �ڽ�������Ӧ��Ļ��Һ���ˣ�ȡ�� �� | �� | ֤������ڳ����� |
��ʵ�鷴˼��
ʵ�鲽����У��Ȼ�����Һ������Ŀ���� ��
 ���������2����β����ô�����Ʒ�и����ʵĺ�����
���������2����β����ô�����Ʒ�и����ʵĺ�����
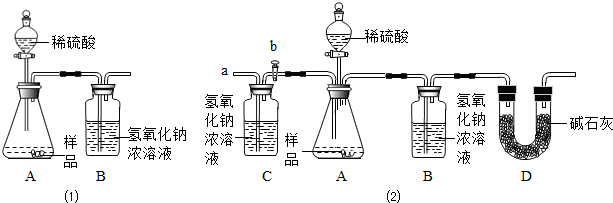
��1��Ϊ�����ô�����Ʒ���ɷֵĺ�����С���������ͼװ�ã�ͨ������Bװ�ö��������仯��ȷ����Ʒ�и��ɷֵĺ�������װ�����������ã�ϡ��������Ʒ������װ��B��CO2��NaOH����ȫ��Ӧ�������������ʵ�鷢�֣��ⶨ�����ƫ����ƫ���ԭ���Т� ��
�� ��
��2��С������ʦ��ָ����������������µ�ʵ��װ�á���ʵ�������²������裺

�ټ��װ�������ԣ���w g �������Ʒ������ƿ�У�
�ڳ���ʢ����������Ũ��Һ��Bƿ������
�۴���b���ӵ���a����������һ�����Ŀ������رջ���b��
������ƿ����μ���ϡ���������ٲ�������
���ٴγ���ʢ����������Ũ��Һ��Bƿ������
����b���ӵ���a����������һ�����Ŀ������رջ���b��
�߸���Bƿ���ӵ�������������Ʒ�и��ɷֵĺ�����
��ʵ�����ȷ������ ��
�𰸣����������ϡ���1���������� ��2��Na2CO3 + Ca(OH)2 == CaCO3��+ 2NaOH
��ʵ��̽����
| ʵ�鲽�� | ʵ������ | ||
| �а�ɫ�������� | Na2CO3 + CaCl2 == CaCO3�� + 2NaCl�� | ||
| �μ�ϡ���ᣨ��������ʯ��ˮ�� | �����ݲ���������Һ����ǣ� |
��ʵ�鷴˼��
������Ʒ��Һ�е�Na2CO3
���������2����1���ٿ����е�CO2����װ��B��������������
�� װ��A�в�����CO2δ��װ��B�е�������������
��2���٢ۢڢܢޢݢ�

 ��У����ϵ�д�
��У����ϵ�д�С��ͬѧ��ͨ��ʵ��̽��ij��ҵ���õİ���ƵĵĴ�����Ʒ�ijɷּ�������
��������⡿�ô�����Ʒ�к�����Щ���ʣ�
����������衿ͨ��������С���������¼��裺
��1��ֻ����Na2CO3 �� ��2������Na2CO3��NaHCO3
���������ϡ�
|
|
NaHCO3 |
Na2CO3 |
|
����ϡ���� |
����� |
�������� |
|
���뱥��ʯ��ˮ |
��Һ����� |
����� |
|
����CaCl2��Һ |
���������� |
��Һ����� |
|
������Һ�����ڣ�������ͨ�����ʯ��ˮ |
����ʯ��ˮ����� |
����ʯ��ˮ�����Ա仯 |
��1�����е������Ϊ ��
��2��������Ӧ�Ļ�ѧ����ʽΪ ��
��ʵ��̽����Ϊ��ȷ��������Ʒ�ijɷ֣�С���������ʵ�鷽��������һ�����С��ʵ�鱨�档
|
ʵ�鲽�� |
ʵ������ |
|
|
��ȡ������Ʒ����ˮ�����������CaCl2��Һ�� |
�� |
�÷�Ӧ�ķ���ʽ�� �� |
|
�ڽ�������Ӧ��Ļ��Һ���ˣ�ȡ��Һ �� |
�� |
֤������ڳ����� |
��ʵ�鷴˼��
ʵ�鲽����У��Ȼ�����Һ������Ŀ���� ��
���������2����β����ô�����Ʒ�и����ʵĺ�����
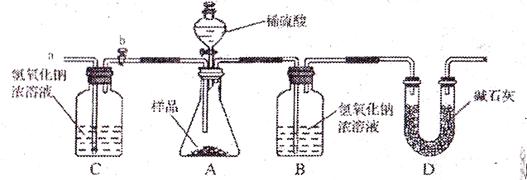
��1��Ϊ�����ô�����Ʒ���ɷֵĺ�����С���������ͼװ�ã�ͨ������Bװ�ö��������仯��ȷ����Ʒ�и��ɷֵĺ�������װ�����������ã�ϡ��������Ʒ������װ��B��CO2��NaOH����ȫ��Ӧ�������������ʵ�鷢�֣��ⶨ�����ƫ����ƫ���ԭ����
 ��
��
��
��
�� ��
��2��С������ʦ��ָ����������������µ�ʵ��װ�á���ʵ�������²������裺
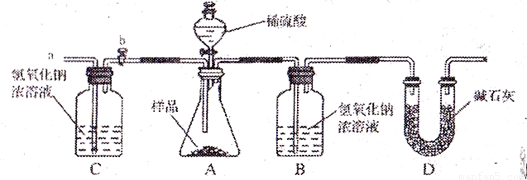
�ټ��װ�������ԣ���w g �������Ʒ������ƿ�У�
�ڳ���ʢ����������Ũ��Һ��Bƿ������
�۴���b���ӵ���a����������һ�����Ŀ������رջ���b��
������ƿ����μ���ϡ���������ٲ�������
���ٴγ���ʢ����������Ũ��Һ��Bƿ������
����b���ӵ���a����������һ�����Ŀ������رջ���b��
�߸���Bƿ���ӵ�������������Ʒ�и��ɷֵĺ�����
��ʵ�����ȷ������ ��
������⣺�ô�����Ʒ�к�����Щ���ʣ�
��������裺ͨ��������С���������¼��裺
��1��ֻ����Na2CO3�� ��2������Na2CO3��NaHCO3
�������ϣ�
| NaHCO3 | Na2CO3 | |
| ����ϡ���� | ����� | �������� |
| ���뱥��ʯ��ˮ | ��Һ����� | ����� |
| ����CaCl2��Һ | ���������� | ��Һ����� |
| ������Һ�����ڣ�������ͨ�����ʯ��ˮ | ����ʯ��ˮ����� | ����ʯ��ˮ�����Ա仯 |
��2��������Ӧ�Ļ�ѧ����ʽΪ ��
ʵ��̽����Ϊ��ȷ��������Ʒ�ijɷ֣�С���������ʵ�鷽��������һ�����С��ʵ�鱨�森
| ʵ�鲽�� | ʵ������ | |
| ��ȡ������Ʒ����ˮ�����������CaCl2��Һ�� | �� | �÷�Ӧ�ķ���ʽ�� �� |
| �ڽ�������Ӧ��Ļ��Һ���ˣ�ȡ��Һ �� | �� | ֤������ڳ����� |
ʵ�鲽����У��Ȼ�����Һ������Ŀ���� ��
�������2����β����ô�����Ʒ�и����ʵĺ�����
��1��Ϊ�����ô�����Ʒ���ɷֵĺ�����С������ˣ�ͼ��1��װ�ã�ͨ������Bװ�ö��������仯��ȷ����Ʒ�и��ɷֵĺ�������װ�����������ã�ϡ��������Ʒ������װ��B��CO2��NaOH����ȫ��Ӧ�������������ʵ�鷢�֣��ⶨ�����ƫ����ƫ���ԭ���Т� ��
�� ��
��2��С������ʦ��ָ���������������ͼ��2����ʵ��װ�ã���ʵ�������²������裺
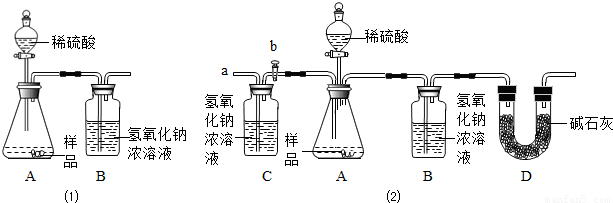
�ټ��װ�������ԣ���w g �������Ʒ������ƿ�У�
�ڳ���ʢ����������Ũ��Һ��Bƿ������
�۴���b���ӵ���a����������һ�����Ŀ������رջ���b��
������ƿ����μ���ϡ���������ٲ�������
���ٴγ���ʢ����������Ũ��Һ��Bƿ������
����b���ӵ���a����������һ�����Ŀ�����
�߸���Bƿ���ӵ�������������Ʒ�и��ɷֵĺ�����
��ʵ�����ȷ������ ��
С��ͬѧ��ͨ��ʵ��̽��ij��ҵ���õİ���ƵĵĴ�����Ʒ�ijɷּ�������
��������⡿�ô�����Ʒ�к�����Щ���ʣ�
����������衿ͨ��������С���������¼��裺
��1��ֻ����Na2CO3 �� ��2������Na2CO3��NaHCO3
���������ϡ�
| NaHCO3 | Na2CO3 | |
| ����ϡ���� | ����� | �������� |
| ���뱥��ʯ��ˮ | ��Һ����� | ����� |
| ����CaCl2��Һ | ���������� | ��Һ����� |
| ������Һ�����ڣ�������ͨ�����ʯ��ˮ | ����ʯ��ˮ����� | ����ʯ��ˮ�����Ա仯 |
��1�����е������Ϊ ��
��2��������Ӧ�Ļ�ѧ����ʽΪ ��
��ʵ��̽����Ϊ��ȷ��������Ʒ�ijɷ֣�С���������ʵ�鷽��������һ�����С��ʵ�鱨�档
| ʵ�鲽�� | ʵ������ | |
| ��ȡ������Ʒ����ˮ�����������CaCl2��Һ�� | �� | �÷�Ӧ�ķ���ʽ�� �� |
| �ڽ�������Ӧ��Ļ��Һ���ˣ�ȡ��Һ �� | �� | ֤������ڳ����� |
��ʵ�鷴˼��
ʵ�鲽����У��Ȼ�����Һ������Ŀ���� ��
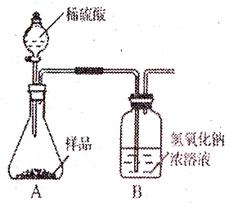 ���������2����β����ô�����Ʒ�и����ʵĺ�����
���������2����β����ô�����Ʒ�и����ʵĺ�����
��1��Ϊ�����ô�����Ʒ���ɷֵĺ�����С���������ͼװ�ã�ͨ������Bװ�ö��������仯��ȷ����Ʒ�и��ɷֵĺ�������װ�����������ã�ϡ��������Ʒ������װ��B��CO2��NaOH����ȫ��Ӧ�������������ʵ�鷢�֣��ⶨ�����ƫ����ƫ���ԭ���Т� ��
�� ��
��2��С������ʦ��ָ����������������µ�ʵ��װ�á���ʵ�������²������裺
�ټ��װ�������ԣ���w g �������Ʒ������ƿ�У�
�ڳ���ʢ����������Ũ��Һ��Bƿ������
�۴���b���ӵ���a����������һ�����Ŀ������رջ���b��
������ƿ����μ���ϡ���������ٲ�������
���ٴγ���ʢ����������Ũ��Һ��Bƿ������
����b���ӵ���a����������һ�����Ŀ������رջ���b��
�߸���Bƿ���ӵ�������������Ʒ�и��ɷֵĺ�����
��ʵ�����ȷ������ ��