��Ŀ����
��Һ�������г��������ʣ����������Ϣ�ش����⣺
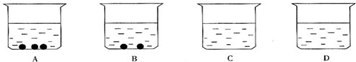
С���ϣ�����ص��ܽ����ֵ������ʾ��
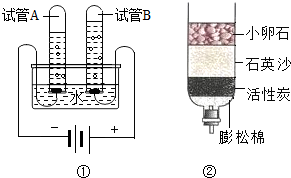
��1���������Һ�е������� ��
��2������A��B��C��D�ĸ��ձ��зֱ�ʢ��50gˮ����40��ʱ�����ĸ��ձ��зֱ����55g��43g��32g��16g����أ�����ܽ����ͼ��ʾ��
�� ��ʢ��һ���DZ�����Һ�� ��ʢ��һ���Dz�������Һ��
��ʹB�ձ�����Һ������50��ʱ����Һ������Ϊ g��
�۲���һ�ַ���ʹA�еĹ���ǡ���ܽ⣬���������ĸ��ձ��е���Һ˵����ȷ���� ��
A���ܼ������������ B���ձ�A�е���Һ���������������
C����������������������ȵ� D����������������ȣ�
С���ϣ�����ص��ܽ����ֵ������ʾ��
| ��Ŀ | 10�� | 20�� | 30�� | 40�� | 50�� | 60�� | 70�� |
| KNO3 | 21g | 32g | 46g | 64g | 86g | 110g | 138g |
��2������A��B��C��D�ĸ��ձ��зֱ�ʢ��50gˮ����40��ʱ�����ĸ��ձ��зֱ����55g��43g��32g��16g����أ�����ܽ����ͼ��ʾ��

��
��ʹB�ձ�����Һ������50��ʱ����Һ������Ϊ
�۲���һ�ַ���ʹA�еĹ���ǡ���ܽ⣬���������ĸ��ձ��е���Һ˵����ȷ����
A���ܼ������������ B���ձ�A�е���Һ���������������
C����������������������ȵ� D����������������ȣ�
��������1�����ʵ��жϣ����塢��������Һ��ʱ�����塢���������ʣ�Һ��ʱ�ܼ�������Һ����ʱ��������ܼ������ٵ������ʣ�ֻҪ��ˮ��ˮһ�����ܼ���
��2����һ���¶Ⱥ��ܼ��У���������������Һ�У����ʴﵽ����ܽ����������ʲ����ܽ⣬����Һһ��Ϊ������Һ��û�й�����������Һ������ǡ�ñ��ͻ��DZ�����Һ������ܽ�ȵĸ����������Һ�뱥����Һ���ת���������ǣ�
��2����һ���¶Ⱥ��ܼ��У���������������Һ�У����ʴﵽ����ܽ����������ʲ����ܽ⣬����Һһ��Ϊ������Һ��û�й�����������Һ������ǡ�ñ��ͻ��DZ�����Һ������ܽ�ȵĸ����������Һ�뱥����Һ���ת���������ǣ�
����⣺��1���������Һ������������أ�
��2�������ݱ�����Һ�ĸ����֪��A��B���й�����֣�һ���DZ�����Һ����40��ʱ����ص��ܽ��64g����֪50gˮ���ܽ�32g�����ǡ�ñ��ͣ�Dһ�������ͣ�
��50��ʱ����ص��ܽ��86g����֪50gˮ���ܽ�43g�����ǡ�ñ��ͣ��ɼ�ʹB�ձ�����Һ������50��ʱ���������ȫ�ܽ⣬��Һ������Ϊ93g��
����������ص��ܽ�����¶ȵ����߶��������Ա�����Һ��Ϊ�����Ϳ��������¶ȣ������ܼ�Ҳ��ʹ������Һ��Ϊ��������Һ������������¶ȵķ�ʹ����ǡ���ܽ⣬���ܼ��������䣬�����������ӣ���Һ���������������Ϊ���¶��µı�����Һ����ʱ��Һ�����������AB��ȷ������ü����ܼ��ķ�ʹ����ǡ���ܽ⣬���ܼ������ʵ����������ӣ���������������������ȵģ���C��ȷ��������ʲô����ʹ����ǡ���ܽ⣬���ʵ������������ӣ�����D����
�ʴ�Ϊ����1������أ�2����ABC��D��93����ABC
��2�������ݱ�����Һ�ĸ����֪��A��B���й�����֣�һ���DZ�����Һ����40��ʱ����ص��ܽ��64g����֪50gˮ���ܽ�32g�����ǡ�ñ��ͣ�Dһ�������ͣ�
��50��ʱ����ص��ܽ��86g����֪50gˮ���ܽ�43g�����ǡ�ñ��ͣ��ɼ�ʹB�ձ�����Һ������50��ʱ���������ȫ�ܽ⣬��Һ������Ϊ93g��
����������ص��ܽ�����¶ȵ����߶��������Ա�����Һ��Ϊ�����Ϳ��������¶ȣ������ܼ�Ҳ��ʹ������Һ��Ϊ��������Һ������������¶ȵķ�ʹ����ǡ���ܽ⣬���ܼ��������䣬�����������ӣ���Һ���������������Ϊ���¶��µı�����Һ����ʱ��Һ�����������AB��ȷ������ü����ܼ��ķ�ʹ����ǡ���ܽ⣬���ܼ������ʵ����������ӣ���������������������ȵģ���C��ȷ��������ʲô����ʹ����ǡ���ܽ⣬���ʵ������������ӣ�����D����
�ʴ�Ϊ����1������أ�2����ABC��D��93����ABC
���������⿼����ѧ���Ա�����Һ���жϼ�������Һ�벻������Һ��ת����Ӧע�������ı仯���������

��ϰ��ϵ�д�
 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�
�����Ŀ

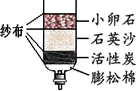
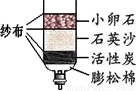
 ˮ������֮Դ������Ӧ���˽�ˮ������ˮ��Դ��
ˮ������֮Դ������Ӧ���˽�ˮ������ˮ��Դ��