��Ŀ����
����Ŀ��(6�֣���֪A��B��C��D��E��F��G��H��M��N��Ϊ���л�ѧ�г��������ʣ�����A��C��D��M��FΪ��������A��C��Ԫ��������ͬ��HΪ�����Լ����X��B�г��ȼ�տ�������ɫ����D��EΪ���ý������ϵ���Ҫ�ɷ֡�������֮������ϵ����ͼ��ʾ�����ַ�Ӧ��������������ʡ������
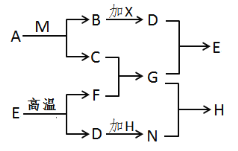
��1������A�Ļ�ѧʽΪ��_________��
��2��д��G��N��Ӧ�Ļ�ѧ����ʽ_____________________________��
��3������F���׳�Ϊ__________������C��Ӧ������Ϊ_____________��
��4����ת��������û���漰���Ļ�����Ӧ����Ϊ_______________��
���𰸡���1��H2O2 ��2��Ca(OH��2+Na2CO3 =CaCO3��+2NaOH
��3����ʯ�� �ų������� ��4���û���Ӧ
��������
�����������1������ͼʽ֪��AΪ H2O2��BΪ������XΪ������̼��FΪ�����ƣ�GΪ�������ƣ�EΪ̼��ƣ�HΪ����������2����ѧ����ʽΪ�� Ca(OH��2+Na2CO3 =CaCO3��+2NaOH��3��F���׳�Ϊ��ʯ������ʯ����ˮ��Ӧ���ų���������4����ת��������û���漰���û���Ӧ��

��ϰ��ϵ�д�
�����Ŀ
