��Ŀ����
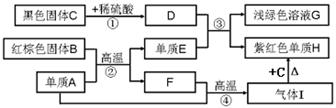
A~G���dz��л�ѧѧϰ���ij������ʣ���Щ������һ�������£�����������ͼ��ʾ��ת����ϵ����֪A��EΪ���ʣ�����Ϊ�������G�Ǵ���ʯ�е���Ҫ�ɷ֡�
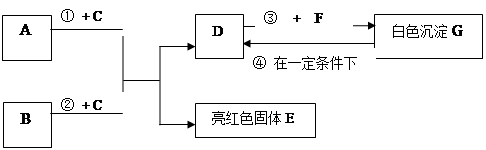
�����ͼʾ�Ʋ⣬�ش��������⣺
��1��B��C�Ļ�ѧʽ�ֱ��� �� ��
��2��F��ˮ��Һ�������������� ��
��3����Ӧ�ٵĻ�ѧ����ʽΪ_____________ ��
��4���ڳ����·����ķ�Ӧ�ܵĻ�ѧ����ʽΪ ____________ ��
��5��50g G��һ��������ת��ΪDʱ�����õ���D�������� g��

�����ͼʾ�Ʋ⣬�ش��������⣺
��1��B��C�Ļ�ѧʽ�ֱ��� �� ��
��2��F��ˮ��Һ�������������� ��
��3����Ӧ�ٵĻ�ѧ����ʽΪ_____________ ��
��4���ڳ����·����ķ�Ӧ�ܵĻ�ѧ����ʽΪ ____________ ��
��5��50g G��һ��������ת��ΪDʱ�����õ���D�������� g��
��1�� CO ��1�֣� CuO ��1�֣�
��2�� Ca2+��OH�C ��1�֣�
��3�� C + 2CuO 2CuO + CO2�� ��2�֣�δ��ƽ��©������©�� ֻ��1�֣�
2CuO + CO2�� ��2�֣�δ��ƽ��©������©�� ֻ��1�֣�
��4�� CaCO3 + 2HCl = CaCl2 + H2O + CO2�� ��2�֣�δ��ƽ��©�� ֻ��1�֣�
��5�� 22 ��2�֣�
��2�� Ca2+��OH�C ��1�֣�
��3�� C + 2CuO
 2CuO + CO2�� ��2�֣�δ��ƽ��©������©�� ֻ��1�֣�
2CuO + CO2�� ��2�֣�δ��ƽ��©������©�� ֻ��1�֣���4�� CaCO3 + 2HCl = CaCl2 + H2O + CO2�� ��2�֣�δ��ƽ��©�� ֻ��1�֣�
��5�� 22 ��2�֣�
����GΪ����ʯ����Ҫ�ɷ֣�����GΪ̼��ƣ���EΪ����ɫ���嵥�ʣ�����Ϊͭ����A��C��Ӧ�õ�ͭ��D��B��C��ӦҲ�ܵõ�ͭ��D����CΪ�������˵��C�Ǻ�ͭ�Ļ�������ݷ�Ӧ�ص�����ж�Ϊ����ͭ������������ԭ��Ӧ����E�ܺ�̼���֮���ת����˵��EΪ������̼����AΪ̼��BΪһ����̼������F��Ϊ�Ϊ�������ƣ�
��1��B�ǻ����CҲ�ǻ�������ܹ����ɶ�����̼��ͭ������BΪһ����̼����CΪ����ͭ���仯ѧʽ�ֱ�Ϊ CO��CuO��
��2��F���������ƣ�������Ϊ�����ӣ�+2�ۣ������������ӣ�-1�ۣ�������Ӧ������Ϊ Ca2+��OH-��
��3����Ӧ���ǵ���A�ͻ�����B��Ӧ���ɶ�����̼��ͭ�����Ե���AΪľ̿����Ӧ�����Ǹ��£���Ӧ�Ļ�ѧ����ʽΪ 2CuO+C 2Cu+CO2����
2Cu+CO2����
��4��̼���ת��Ϊ������̼;������Ψһ�ģ�������Ŀ�������ڳ����·���������Ӧ����̼��������ᷴӦ�����Ȼ��ƺ�ˮ�Լ�������̼����Ӧ�ķ���ʽΪ CaCO3+2HCl=CaCl2+CO2��+H2O��
��5��50g ̼�����һ��������ת��Ϊ������̼�������ɵĶ�����̼������Ϊx������������;����һ���ӵ�̼��ƾͶ�Ӧһ���ӵĶ�����̼�����Դ˴������ù�ϵʽ����
CaCO3 ��������CO2
100 44
50g x
100/44 =50g/x�� x=22g
�𣺵õ��Ķ�����̼�������� 22 g��
��1��B�ǻ����CҲ�ǻ�������ܹ����ɶ�����̼��ͭ������BΪһ����̼����CΪ����ͭ���仯ѧʽ�ֱ�Ϊ CO��CuO��
��2��F���������ƣ�������Ϊ�����ӣ�+2�ۣ������������ӣ�-1�ۣ�������Ӧ������Ϊ Ca2+��OH-��
��3����Ӧ���ǵ���A�ͻ�����B��Ӧ���ɶ�����̼��ͭ�����Ե���AΪľ̿����Ӧ�����Ǹ��£���Ӧ�Ļ�ѧ����ʽΪ 2CuO+C
 2Cu+CO2����
2Cu+CO2������4��̼���ת��Ϊ������̼;������Ψһ�ģ�������Ŀ�������ڳ����·���������Ӧ����̼��������ᷴӦ�����Ȼ��ƺ�ˮ�Լ�������̼����Ӧ�ķ���ʽΪ CaCO3+2HCl=CaCl2+CO2��+H2O��
��5��50g ̼�����һ��������ת��Ϊ������̼�������ɵĶ�����̼������Ϊx������������;����һ���ӵ�̼��ƾͶ�Ӧһ���ӵĶ�����̼�����Դ˴������ù�ϵʽ����
CaCO3 ��������CO2
100 44
50g x
100/44 =50g/x�� x=22g
�𣺵õ��Ķ�����̼�������� 22 g��

��ϰ��ϵ�д�
�����Ŀ