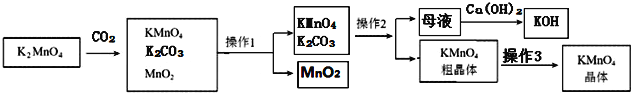
��Ŀ����
����Ŀ��Ϊ̽������������Һ��ϡ���ᷴӦ��������Һ�����ʵijɷ֣�С����ͬѧ��ͨ���ⶨ��Һ��pH���õ�����ͼ��ʾ��pH�仯���ߣ���ش�

��1����ͼ������������ġ���Һ����ָ�������ʻ�ѧʽ��_____��b��ĺ�����_____��
��2���÷�Ӧ�Ļ�ѧ����ʽΪ_____��
��3��ȡ��Ӧ�����е�����Һ��M���Թ��У��μ�Na2CO3��Һ�����ȹ۲쵽�����ݲ�����һ��ʱ�����ְ�ɫ�������ɴ��Ʋ⣬M��Һ�к��е��������У�д���ӷ��ţ�_____��
���𰸡�HCl �����ϡ��������������ǡ����ȫ��Ӧ Ca(OH)2+2HCl=CaCl2+2H2O H+��Ca2+
��������
��1����ͼ��֪����Һ��pH��С��������ʵ���ǽ�ϡ������뵽����������Һ�У������������������Ǽ���ϡ�����������ϡ����Ļ�ѧʽΪHCl������HCl��
��ͼ��֪����Һ��pHΪ7����Һ�����ԣ�������ϡ��������������ǡ����ȫ��Ӧ����������ϡ��������������ǡ����ȫ��Ӧ��
��2����Ӧ�У��������������ᷴӦ�����Ȼ��ƺ�ˮ���ʷ�Ӧ�Ļ�ѧ����ʽдΪ��Ca(OH)2+2HCl=CaCl2+2H2O��
��3��ȡ��Ӧ�����е�����Һ��M���Թ��У��μ�Na2CO3��Һ�����ȹ۲쵽�����ݲ���������Ϊ��Ӧ����Һ�в�����ϡ������̼���Ʒ�Ӧ�����˶�����̼��һ��ʱ�����ְ�ɫ����������Һ�е��Ȼ�����̼���Ʒ�Ӧ������̼��Ƴ�������������˵��M��Һ�е��������������Ȼ��ƣ���������Һ�н������������Ϊ�����ӣ��Ȼ�������Һ�н�������������������ӣ������ӵĸ�ΪH+�������ӵķ���ΪCa2+������H+��Ca2+��

 ��ĩ1�����ʽ���������ϵ�д�
��ĩ1�����ʽ���������ϵ�д�����Ŀ����ͼ�Ǿ������ȼ����ˮ��ʾ��ͼ�����ݴ�ͼ�ش�
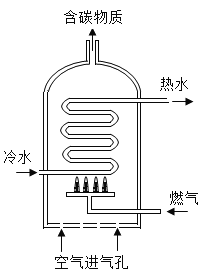
��1��ij��ˮ������Ȼ��Ϊȼ���� ����ȫȼ��3.2kg�ļ��飬����������������Ϊ______kg��
��2�����±���1kg���ֲ�ͬȼ��ȼ�ղ���CO2��SO2���������ɱ������ݿ�֪����Ȼ���DZ�ú��������Դ��ԭ����______��
ȼ�� | ȼ�ղ�������/g | |
CO2 | SO2 | |
���� | 2900 | 5.0 |
��Ȼ�� | 2500 | 0.1 |
ú | 2500 | 11.0 |