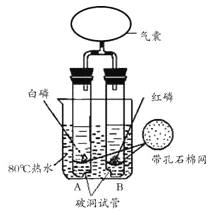
��Ŀ����
����Ŀ��������̼���������ã���һ����ˮһ����Ҫ����Դ���ۺ����ö�����̼Ҫ���ݵ���Ҫԭ��ֱ��У�
��1������ԭ����ٳ���̼�����һ��ʵ��Ϊ_________��
��2������ԭ��ѧ�������о�������Ķ�����̼��������һ�������·�Ӧת���ɼ����ˮ����д���÷�Ӧ�Ļ�ѧ����ʽ��_________��
��3������ԭ��Ŀǰʹ��Ĥ���뷨�ӿ����з����CO2��������̶�����̼��������_______������������������ѧ�����仯����ЩCO2����Ϊ������ϣ���������______�����˹����ꡣ
���𰸡����ֹصơ��̿���ѭ��ʹ�á�����һ���Բ;ߡ��ٿ�������ɫ���е� CO2+4H2 CH4+2H2O ���� �ɱ�
CH4+2H2O ���� �ɱ�
��������
��1������ԭ�����ֹصơ��̿���ѭ��ʹ�á�����һ���Բ;ߡ��ٿ�������ɫ���еȶ����ϵ�̼���
��2������ԭ������̼��������һ�������·�Ӧת���ɼ����ˮ����Ӧ�Ļ�ѧ����ʽ�ǣ�CO2+4H2 CH4+2H2O
CH4+2H2O
��3������ԭ��Ŀǰʹ��Ĥ���뷨�ӿ����з����CO2�����������û�������ʲ�����������̼�������������仯����ЩCO2����Ϊ������ϣ���������ɱ����ɱ������������������˹����ꡣ

����Ŀ��ij�����������Ĵ����Ʒ�к��������Ȼ������ʣ����Ʒ��װ����ע����̼���ơ�93%��Ϊ�ⶨ�ò�Ʒ�к�̼���Ƶ���������������������ʵ�飺ȡ12.0g������Ʒ�����ձ��У��Ƶ��ձ�����ʢ������Ʒ��������Ϊ158.0g���ٰ�120gϡ����ƽ���ֳ��ķ����μ�����Ʒ�У�ÿ�ξ���ַ�Ӧ��ʵ�����ݼ�¼���£�
��������Ĵ��� | ��һ�� | �ڶ��� | ������ | ���Ĵ� |
ϡ���������/g | 30 | 30 | 30 | 30 |
�ձ�����ʢ����������/g | 186.2 | 214.4 | 243.6 | 273.6 |
����ݴ˷������㣺
��1����_____�μ����ϡ������ȫ��Ӧ�ˡ�
��2���ò�Ʒ��̼���Ƶ����������Ƿ�ϸ�___��Ҫ��д��������̣������ȷ��0.1%��