��Ŀ����
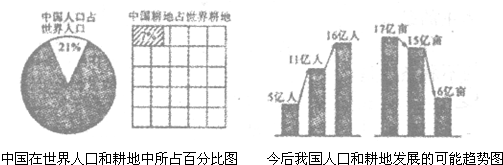
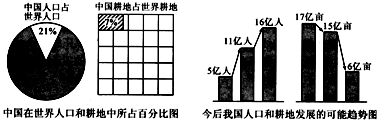
���й��������˿ں�������ռ�ٷֱ�ͼ�ͽ���ҹ��˿ں��ط�չ��������ͼ���ش��������⣺
���ϣ��ҹ�ɭ�����ÿ�걻��ռԼ50���꣬�ݳ�����ÿ��133������ٶ��˻�������ɳĮ�������1��������������500���꣬����90%���Ŀ��ķ����ȷ�����ɵģ������ҹ��ò�������ɹ�ȥ��8243�����½���7958���꣮
��1��Ŀǰ�ҹ���ռ����
��2������ҹ��˿ڷ�չ�Ŀ���������
��3��Ŀǰ���ҶԽ��������������ȡ�����ߺʹ�ʩ�����˿ڷ��棬��ʵ�С�
��4����ɲ���2��ɭ�֡��ݵ���Դ���ٵ���Ҫԭ����
������������Դ�������������������Ȼ��Դ������뿪�����أ����Ǿ������й�ҵ��ũҵ����ͨ����ҵ�Ⱦ��û���ҹ�½�����Լ960��ƽ��ǧ�ף�����������оӵ�3λ�������ҹ��˿��ڶ࣬ƽ��ÿ��ռ�е��������Լ�൱�������˾����������1/3��
����⣺��1����ͼ��Ŀǰ�ҹ���ռ����7%�ĸ���������ռ����21%���˿ڣ�
��2���ҹ��˿ڻ����������˿��������������ӣ��˾����ر仯���������������٣�
��3������ҹ���Դ��״�����˿ڷ��棬��ʵ�С��ƻ���������Ϊһ��������ߣ��ڸ��ط��棬�ѡ�ʮ����ϧ�ͺ�������ÿһ�����أ���ʵ�������ء���Ϊһ��������ߣ�
��4�������������ȷ��������ȿ���������ɳĮ�������ʹ��̬�������϶ȣ���Σ����̬��ȫ����ɳ��������Ƶ�����֣������̬������������״���ɲ�ȡ�Ĵ�ʩ�н�ֹ���ȷ�����ֲ�����֡��̻���ɽ���˸����ֻ��ݡ���������ˮ��Դ��������Դ�ȣ�
�ʴ�Ϊ����1��7��21����2���������������٣���3���ƻ�������ʮ����ϧ�ͺ�������ÿһ�����أ���ʵ�������أ���4���Ŀ��ķ����ȷ�������ֹ���ȷ�����ֲ�����֡��̻���ɽ���˸����ֻ��ݡ���������ˮ��Դ��������Դ�ȣ�
��2���ҹ��˿ڻ����������˿��������������ӣ��˾����ر仯���������������٣�
��3������ҹ���Դ��״�����˿ڷ��棬��ʵ�С��ƻ���������Ϊһ��������ߣ��ڸ��ط��棬�ѡ�ʮ����ϧ�ͺ�������ÿһ�����أ���ʵ�������ء���Ϊһ��������ߣ�
��4�������������ȷ��������ȿ���������ɳĮ�������ʹ��̬�������϶ȣ���Σ����̬��ȫ����ɳ��������Ƶ�����֣������̬������������״���ɲ�ȡ�Ĵ�ʩ�н�ֹ���ȷ�����ֲ�����֡��̻���ɽ���˸����ֻ��ݡ���������ˮ��Դ��������Դ�ȣ�
�ʴ�Ϊ����1��7��21����2���������������٣���3���ƻ�������ʮ����ϧ�ͺ�������ÿһ�����أ���ʵ�������أ���4���Ŀ��ķ����ȷ�������ֹ���ȷ�����ֲ�����֡��̻���ɽ���˸����ֻ��ݡ���������ˮ��Դ��������Դ�ȣ�
���������⿼���ҹ����������д��ڵ����⼰��ȡ����Ӧ��ʩ��Ҫ�μǣ�

��ϰ��ϵ�д�
 �̲�ȫ���ִʾ�ƪϵ�д�
�̲�ȫ���ִʾ�ƪϵ�д�
�����Ŀ