��Ŀ����
�����Ϸ�����ͼ�����ش�����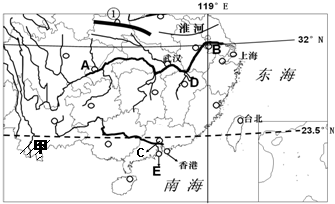
��1��B������
��2��E���ر�����������֧����ҵ��
��3����
��4��ͼʾ������ֲ����Ҫ��ʳ������
��5��ͼ�м������ҹ�����ɫ������
�������μ��ҹ��������������ɵó��𰸣�
����⣺��ͼ��֪����1��B�����ǽ���ʡ���������ģ�D���ҹ�������ĵ�ˮ��۶��������2��E���ر�����������֧����ҵ�Dz�������ҵ��������۵ľ��������������У���3�����������ҹ�1��0������ߡ�800���Ƚ�ˮ���߾����ĵ�������4��ͼʾ������ֲ����Ҫ��ʳ������ˮ������Ҫ�����������Ͳˣ���Ҫ��ֲ�����������ȴ�������Ҷ�֣���5��ͼ�м������ҹ�����ɫ��������˫���ɣ��õ����������������Ǵ��壮 �������⣮
�ʴ�Ϊ����1�����գ� ۶������2���������Σ� ���ڣ���3�����룻��4��ˮ���� �Ͳˣ� ���ȴ�������Ҷ�֣���5����˫���ɣ� ���壮
�ʴ�Ϊ����1�����գ� ۶������2���������Σ� ���ڣ���3�����룻��4��ˮ���� �Ͳˣ� ���ȴ�������Ҷ�֣���5����˫���ɣ� ���壮
������������Ҫ�������ҹ����������������ڻ����⣬ѧ��Ӧ�μ���ػ���֪ʶ��

��ϰ��ϵ�д�
 ��ĩ�����ϵ�д�
��ĩ�����ϵ�д�
�����Ŀ
����ͼ���ش��⡣
��С��1������ƺӱ۰���̫��������̤ǧ������������ꡮ�ڽ𡯡����������ĵ���λ��
| A���������� | B��������ԭ |
| C�����ľ��� | D����ظ�ԭ |
| A��200mm��Ƚ�ˮ���� |
| B����������Ǽ������ķֽ��� |
| C�������������Ϸ������ķֽ��� |
| D�����Ƶ�һ��������ڶ������ݵķֽ��� |
| A������Դͷ | B������ | C������ | D������ |
| A����ȡֲ���ֲ��뽨����ȴ�ʩ���ϣ����ˮ������ |
| B��ȫ���˸����ֻ��� |
| C��������ʳ����������Ѷ��¿���Ϊ���� |
| D�������������ݣ�ʵ����Ȼ���� |
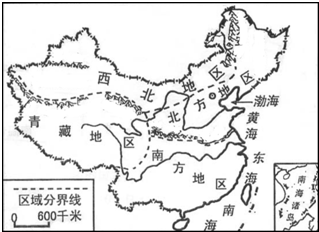
����ͼ���ش��⡣
��С��1���õؽ�����Ϊƽ������������ص����Ҫԭ����
| A�������� | B�����¸� | C������ǿ | D����ˮ�� |
| A������������ | B���齭�������� | C���Ϸ������� | D���������� |
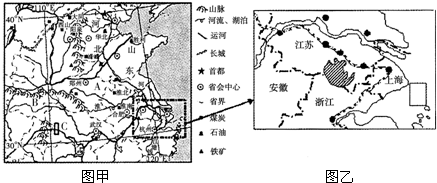
 ���ҹ��Ĵ��������ͼ������������⣮
���ҹ��Ĵ��������ͼ������������⣮