��Ŀ����
��2008?�Ϻ����������������桱�ܷ�ӳ���еĿ�������״�����±���2008��ij���ҹ��������ֳ��еĿ����������棬���С�������Ⱦָ�����Ƿ�ӳ�����ۿ���������ָ�꣮�����ش�
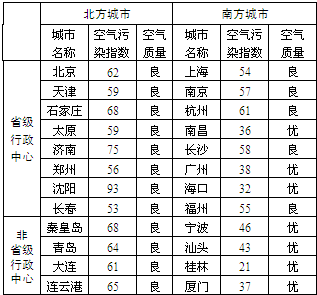
��1�����ձ��С�������Ⱦָ�����͡��������������Կ�����������Ⱦָ��ԽС����������Խ
��2�������Ϸ����кͱ��������У����������Ϻõ���
��3��д��һ����ɴ�����Ⱦ����Ҫԭ��

��1�����ձ��С�������Ⱦָ�����͡��������������Կ�����������Ⱦָ��ԽС����������Խ
��
��
���ڱ��г����У����տ���������õij���������
����
����2�������Ϸ����кͱ��������У����������Ϻõ���
�Ϸ�
�Ϸ�
���У�ʡ���������ĺͷ�ʡ�����������У���������Ϊ���š��ij���������Խ϶������ʡ����������
��ʡ����������
����3��д��һ����ɴ�����Ⱦ����Ҫԭ��
��ҵ�����ŷŴ�������
��ҵ�����ŷŴ�������
��������������Ⱦָ��ָ��������Ⱦ�ij̶ȣ�ָ��Խ������ȾԽ���أ���������Ⱦָ��С��50ʱ����������������һ������������״�����ţ���������Ⱦָ����51-100֮��ʱ���������������Ƕ�������������״��������
����⣺��1�����ձ��С�������Ⱦָ�����͡��������������Կ�����������Ⱦָ��ԽС����������Խ�ã��ڱ��г����У����տ���������õij����ǹ��֣�
��2�������Ϸ����кͱ��������У����������Ϻõ����Ϸ����У�ʡ���������ĺͷ�ʡ�����������У���������Ϊ���š��ij���������Խ϶���Ƿ�ʡ���������ģ�
��3����ɴ�����Ⱦ����Ҫԭ���й�ҵ�����ŷŴ��������ȣ�
�ʴ�Ϊ��
��1���ã����֣�
��2���Ϸ�����ʡ���������ģ�
��3����ҵ�����ŷŴ���������
��2�������Ϸ����кͱ��������У����������Ϻõ����Ϸ����У�ʡ���������ĺͷ�ʡ�����������У���������Ϊ���š��ij���������Խ϶���Ƿ�ʡ���������ģ�
��3����ɴ�����Ⱦ����Ҫԭ���й�ҵ�����ŷŴ��������ȣ�
�ʴ�Ϊ��
��1���ã����֣�
��2���Ϸ�����ʡ���������ģ�
��3����ҵ�����ŷŴ���������
���������⿼��������������ۣ�������ɣ�

��ϰ��ϵ�д�
�����Ŀ