��Ŀ����
�����ҹ���������ͼ����ͼ1�����ش����⣮
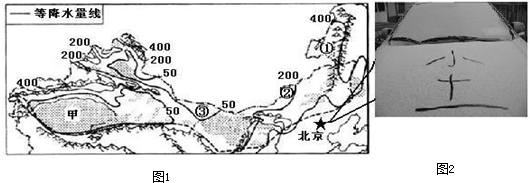
��1���ܽ�ˮ�ֲ����ɵ�Ӱ�죬�����Ӣٵ����ٵ��ۣ�ֲ����������Ϊ ����Į��ԭ����Į���ڢٵ����ܼ��������������� ����������ϸë����ţ����
��2����������ҹ����������� �ӣ�������зḻ��������Դ�����ҹ������������㣬���������������Դ��ԭ���� ��
A������ḻ B�����ȳ��� C�����ؽ�ȱ D����Դȱ��
��3������������ũҵ�� Ϊ��������ҵ����ҵ����ֲҵ����
��4������������Ȼ��������Ҫ������ �������ԭ���� ��
��5��2013��3��20�գ�����ӭ�����봺������Ϊ���ص�ɳ��������ͼ2������������������3��20�յ������������ ��

��6�������л���������ĵļ��������ʾ���ɹŹ����ҹ����ɹ��в���ɳ����ʼ���������������ӣ���Ӱ�챱���У������ܶ����������������Ũ���ѽӽ���ﵽ1000��/�����ף���������״���ﵽ������Ⱦ��������ɳ����������Ҫ��ʩ�� ��
��7���½����ηֲ�����������ص��� ��

��1���ܽ�ˮ�ֲ����ɵ�Ӱ�죬�����Ӣٵ����ٵ��ۣ�ֲ����������Ϊ
��2����������ҹ�����������
A������ḻ B�����ȳ��� C�����ؽ�ȱ D����Դȱ��
��3������������ũҵ��
��4������������Ȼ��������Ҫ������
��5��2013��3��20�գ�����ӭ�����봺������Ϊ���ص�ɳ��������ͼ2������������������3��20�յ������������

��6�������л���������ĵļ��������ʾ���ɹŹ����ҹ����ɹ��в���ɳ����ʼ���������������ӣ���Ӱ�챱���У������ܶ����������������Ũ���ѽӽ���ﵽ1000��/�����ף���������״���ﵽ������Ⱦ��������ɳ����������Ҫ��ʩ��
��7���½����ηֲ�����������ص���
�������½��ĵ��α�����س�Ϊ����ɽ�����衱�����еġ���ɽ��ָ���ǰ���̩ɽ����ɽ������ɽ������ָ����������غ�����ľ�����أ��ҹ��������������ľ��أ���������ҹ��������������ľ�ӣ�����������Ȼ�����ص��Ǹɺ���
����⣺��1���ܽ�ˮ�ֲ����ɵ�Ӱ�죬�����Ӣٵ����ٵ��ۣ�ֲ����������Ϊ��ԭ����Į��ԭ����Į����ԭ���Ǿ��뺣��Խ��ԽԶ����ˮԽ��Խ�٣��ڢٵ����ܼ���������������������������ţ��
��2������ؼ�����ľ��أ���������ҹ���������������ľ�ӣ�������зḻ��������Դ�����ҹ������������㣬���������������Դ��ԭ������Դȱ����
��3������������ũҵ������ҵΪ����
��4������������Ȼ��������Ҫ�����Ǹɺ��������ԭ���������½���ຣңԶ����ˮϡ�٣�
��5����������ĸ�ѡ��ɵã�A��ʾ��������B��ʾ���DZ��꣬C��ʾ����ɳ������D��ʾ�������ꣻ
��6������ɳ����������Ҫ��ʩ��ֲ���ֲݣ�
��7���½����ηֲ�����������ص�����ɽ�����裻
�ʴ�Ϊ��
��1����ԭ����������
��2������ľ��D��
��3������ҵ��
��4���ɺ��������½���ຣңԶ����ˮϡ�٣�
��5��C��
��6��ֲ���ֲݣ�
��7����ɽ�����裮
��2������ؼ�����ľ��أ���������ҹ���������������ľ�ӣ�������зḻ��������Դ�����ҹ������������㣬���������������Դ��ԭ������Դȱ����
��3������������ũҵ������ҵΪ����
��4������������Ȼ��������Ҫ�����Ǹɺ��������ԭ���������½���ຣңԶ����ˮϡ�٣�
��5����������ĸ�ѡ��ɵã�A��ʾ��������B��ʾ���DZ��꣬C��ʾ����ɳ������D��ʾ�������ꣻ
��6������ɳ����������Ҫ��ʩ��ֲ���ֲݣ�
��7���½����ηֲ�����������ص�����ɽ�����裻
�ʴ�Ϊ��
��1����ԭ����������
��2������ľ��D��
��3������ҵ��
��4���ɺ��������½���ຣңԶ����ˮϡ�٣�
��5��C��
��6��ֲ���ֲݣ�
��7����ɽ�����裮
���������⿼��֪ʶ��϶࣬��ǿα�֪ʶ���ͼ���ɣ�

��ϰ��ϵ�д�
 �����ҵ��ٿ���������������ϵ�д�
�����ҵ��ٿ���������������ϵ�д�
�����Ŀ
 ���ҹ���������ͼ���ش�
���ҹ���������ͼ���ش�
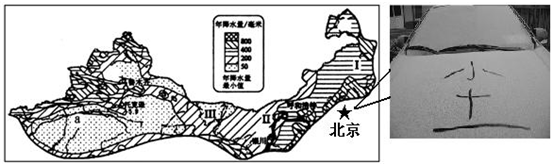
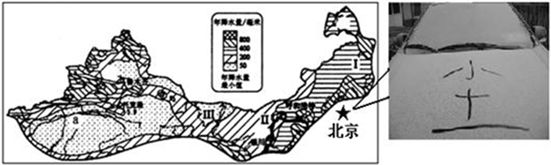
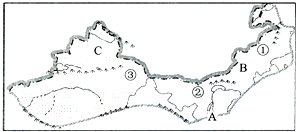 �����ҹ���������ͼ�����ش����⣮
�����ҹ���������ͼ�����ش����⣮