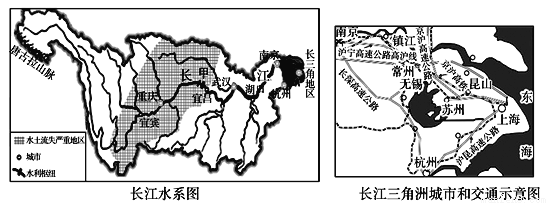
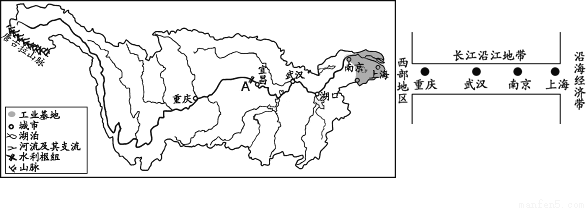
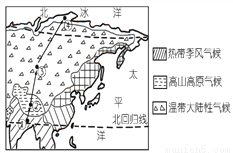
��Ŀ����
�����纣½�ֲ�ͼ���ش����и��⡣

1.����ͼ�д�������������ȷ����
A. ������ӵĴ����Ǣ� B. �ܢݴ��ķֽ���������ʿ�˺�
C. �����С�Ĵ��ޢ� D. ���Ĵ��ޢ�
2.�����������ȣ��С��ȴ���½��֮�ƵĴ�������ͼ�е�
A. �� B. �� C. �� D. ��
1.B 2.C �������� 1.��ͼ��֪�����ޢ�Ϊ���ޣ���Ϊŷ�ޣ���Ϊ���ޣ���Ϊ�����ޣ����������ޡ����ޢٵ����Ը�ԭɽ��Ϊ�������θ��ӣ�ѡ��A��ȷ���ܢݴ��ķֽ����ǰ������˺ӣ���B����ȷ�������С�Ĵ��ޢ����ޣ���ȷ�����Ĵ��ޢ��ϼ��ޣ���D��ȷ���ʸ�������ѡB�� 2.��ͼ��֪���۷�����λ���ϱ��ع���֮�䣬��λ���ȴ����С��ȴ���½��֮�ơ���ѡC��
��ϰ��ϵ�д�
 ��ս100��Ԫ����Ծ�ϵ�д�
��ս100��Ԫ����Ծ�ϵ�д� ������ϵ�д�
������ϵ�д�
�����Ŀ