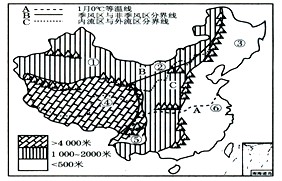
��Ŀ����
 �����й�����ͼ��������ͼ����Ϣ������и��⣺
�����й�����ͼ��������ͼ����Ϣ������и��⣺��1���ҹ����ƣ��Ժ��������Ӱ���ǣ�
����������������
����������������
��2��A �߾�����ɽ����
����
����
������������
����
�������ϱ�����ĸ������������Բ��죬�ϲ���ˮ��
ˮ��
��������������
����
Ϊ������3��B�ߵĶ���һ����
�ļ���
�ļ���
Ӱ�����ԣ����Գ�Ϊ�������������Ӱ���ǵ����ҹ���ˮʱ�շֲ���������Ҫԭ�����ش�A���ܼ���Ӱ�죬�ҹ�����������ˮ��Ҫ������
����
����
���ڣ�B���ҹ��꽵ˮ���ռ�ֲ����������Ǵ�
�����غ�
�����غ�
��������½
������½
�ݼ���C���ļ������ȶ�������
����
����
�ֺ�����4��C���������ĺ�����Ϊ
������
������
��ũҵ����������
����
ҵΪ������ֲҵΪ���
���
ũҵ����5���ϵ�����ʱ����ʦ��ͬѧ�ǽ���������ᣮС����С����С����С��ֱ�̸�˸��Ե�������ܣ����ܲ³�����������ȥ��ͼ�е��ĸ��ط������ͼ����ȷ�ı�����ں���ĺ����ϣ�
С�������紩�ް��紩ɴ��Χ�Ż�¯�����ϣ���
��
��
С��������÷ʱ�ڼҼ��꣬��ݳ��������ܣ���
��
��
С�����Բԣ�Ұãã���紵�ݵͼ�ţ��
��
��
С������һɽ���ļ���ʮ�ﲻͬ�죮��
��
��
���������ҹ����ƶԺ��������Ӱ�죻�Ϸ���������Ȼ����������������ҹ���ˮ��Ӱ�죻�ҹ����ص���Ȼ���������������Ӱ�죻��������֪ʶ�������⣮
����⣺��1���ҹ��������߶��ͣ������ҹ��������������������
��2��A�������뻴��һ�ߣ����ҹ��Ϸ��������ķֽ��ߣ������Ա���������������ԭ���¸�������Ҳ��ͬ�������Ա�����չ���أ��������ϣ���չˮ�
��3��B����һϵ�иߴ��ɽ�����赲�����Ժ����ʪ������������½���γ����ҹ��������ͷǼ������ķֽ��ߣ����߶��ϲ����ļ���Ӱ�����ԣ���ˮƫ���γɼ������������Ӱ�쵼���ҹ���ˮʱ�շֲ�������A���ܼ���Ӱ�죬�ҹ������������^�ڶവ��������ļ��磬��ˮƫ�࣬���������ڣ����������Դ�½�ڲ�����ˮƫ�٣�B���ҹ��Զ����غ���������½���ļ���Ӱ����С�������ҹ��꽵ˮ���ռ�ֲ����������ǴӶ����غ���������½�ݼ���C���ļ���ǿ�����ȶ������½�ˮʱ�ղ����ȣ����ֺ����ֺ���
��4��C�����ҹ��������������ķֽ��ߣ�����������������Ϊ�����ӣ����ڽ�ˮ�٣�ũҵ����������ҵΪ������ֲҵ�Թ��ũҵΪ����
��5��������Ȼ���������������Ӱ�죬���¸��ص���Ȼ��������ǵ�����ϰ�߶��ͬ��С�������紩�ް��紩ɴ��Χ�Ż�¯�����ϣ���������������½���´���½����������ҹ�²��Ӧλ���Ǣ٣�С��������÷ʱ�ڼҼ��꣬��ݳ��������ܡ������ڽ�������������������·ݣ���Ӧ�����Ǣޣ�С�����Բԣ�Ұãã���紵�ݵͼ�ţ����ʫ����������һ���ʲ�ԭ�ľ����ҹ�������õ�����Ӧ�������ɹ���������Ӧ�����Ǣڣ�С������һɽ���ļ���ʮ�ﲻͬ�죮�����־�������ں��ɽ���������ֲ��������ֱ�仯����Ӧ�����Ǣݣ�
�ʴ�Ϊ����1������������������ ��2�����룻 ���ӣ� ˮ� ���أ���3���ļ��磻 ��� �����غ��� ������½�� ���ԣ���4�������ӣ� ������ ��ȣ� ��5���٣��ޣ��ڣ��ݣ�
��2��A�������뻴��һ�ߣ����ҹ��Ϸ��������ķֽ��ߣ������Ա���������������ԭ���¸�������Ҳ��ͬ�������Ա�����չ���أ��������ϣ���չˮ�
��3��B����һϵ�иߴ��ɽ�����赲�����Ժ����ʪ������������½���γ����ҹ��������ͷǼ������ķֽ��ߣ����߶��ϲ����ļ���Ӱ�����ԣ���ˮƫ���γɼ������������Ӱ�쵼���ҹ���ˮʱ�շֲ�������A���ܼ���Ӱ�죬�ҹ������������^�ڶവ��������ļ��磬��ˮƫ�࣬���������ڣ����������Դ�½�ڲ�����ˮƫ�٣�B���ҹ��Զ����غ���������½���ļ���Ӱ����С�������ҹ��꽵ˮ���ռ�ֲ����������ǴӶ����غ���������½�ݼ���C���ļ���ǿ�����ȶ������½�ˮʱ�ղ����ȣ����ֺ����ֺ���
��4��C�����ҹ��������������ķֽ��ߣ�����������������Ϊ�����ӣ����ڽ�ˮ�٣�ũҵ����������ҵΪ������ֲҵ�Թ��ũҵΪ����
��5��������Ȼ���������������Ӱ�죬���¸��ص���Ȼ��������ǵ�����ϰ�߶��ͬ��С�������紩�ް��紩ɴ��Χ�Ż�¯�����ϣ���������������½���´���½����������ҹ�²��Ӧλ���Ǣ٣�С��������÷ʱ�ڼҼ��꣬��ݳ��������ܡ������ڽ�������������������·ݣ���Ӧ�����Ǣޣ�С�����Բԣ�Ұãã���紵�ݵͼ�ţ����ʫ����������һ���ʲ�ԭ�ľ����ҹ�������õ�����Ӧ�������ɹ���������Ӧ�����Ǣڣ�С������һɽ���ļ���ʮ�ﲻͬ�죮�����־�������ں��ɽ���������ֲ��������ֱ�仯����Ӧ�����Ǣݣ�
�ʴ�Ϊ����1������������������ ��2�����룻 ���ӣ� ˮ� ���أ���3���ļ��磻 ��� �����غ��� ������½�� ���ԣ���4�������ӣ� ������ ��ȣ� ��5���٣��ޣ��ڣ��ݣ�
���������⿼�����ҹ����ƶԺ�����Ӱ�죬�Լ�����������ҹ���ˮ��������ũҵ��Ӱ�죬�ҹ����ص���Ȼ���������������Ӱ�죬����ȫ�棬�ѶȽϴ�

��ϰ��ϵ�д�
 ��ڽ��ȫ������ϵ�д�
��ڽ��ȫ������ϵ�д�
�����Ŀ
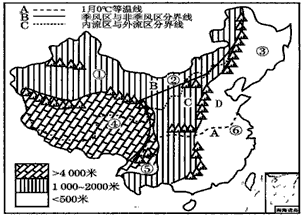
 �����й�����ͼ��������ͼ����Ϣ������и��⣮
�����й�����ͼ��������ͼ����Ϣ������и��⣮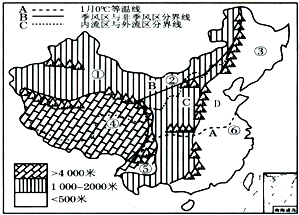 �����й�����ͼ��������ͼ����Ϣ������и��⣮
�����й�����ͼ��������ͼ����Ϣ������и��⣮