��Ŀ����
 ����������ֲ�ͼ�Ϳ����Դ�ֲ�ͼ���ش��������⣺
����������ֲ�ͼ�Ϳ����Դ�ֲ�ͼ���ش��������⣺
��1����������ɳĮ������зḻ��______�����Դ�����ϷǸ�ԭ�ϣ�����зḻ��______��______�����Դ��
��2��A��B���ض�λ�ڳ��������A��Ϊ���ȶ�����ȴ���������B��ȴ���ȴ���ԭ��������Ҫԭ����______��
��3����ͼ�п��Կ��������������ڷֲ��Ͼ���______�ص㣮
��4��ԭ�����硰��ȻҰ��������֮�ƣ���������������ص��ο�ǰ�����ͣ������о������ڸ������Ұ���������4�֣���______��
��5�������Ͼ��÷�չˮƽ��͵Ĵ��ޣ������ԭ����______��Ŀǰ������չ���õ�����������______��
�⣺��1����������ɳĮ������зḻ��ʯ�Ϳ����Դ�����ϷǸ�ԭ�ϣ�����зḻ�Ľ��ʯ��ͭ��ȿ����Դ��
��2��A��B���ض�λ�ڳ��������A��Ϊ���ȶ�����ȴ���������B��ȴ���ȴ���ԭ��������Ҫԭ���Ǻ��θߣ�
��3����ͼ�п��Կ��������������ڷֲ��Ͼ��жԳ��ص㣮
��4�������ڷ����ȴ����ԭ�ϵĶ�����ʨ�ӡ�����������¹������ȣ�
��5�������������Ͼ��÷�չˮƽ��͵Ĵ��ޣ������ԭ���dz��ڵ�ֳ��ͳ�Σ�Ŀǰ������չ���õ�������������Դ�ḻ��
�ʴ�Ϊ��
��1��ʯ�ͣ����ʯ��ͭ��
��2�����θߣ�
��3���Գƣ�
��4��ʨ�ӡ�����������¹������
��4�����ڵ�ֳ��ͳ�Σ���Դ�ḻ��
�����������������½���в����ϱ��ع��ߴ��������ϱ����࣬���Է����ȴ��������Գ��Ϊ���ķֱ�Ϊ�ȴ����֡��ȴ���ԭ���ȴ�ɳĮ�����к������Ŀ����Դ����࣬����������ʯ���ƽ𡢸�����������εȵĴ����Ͳ�����������ǰ�У����ڳ��ڵ�ֳ��ͳ�Σ����ľ��÷�չ�ĺܻ�����������ᾭ�÷�չ������ʮ�ּ�ޣ�
���������⿼�������Դ��Ҫ�μǣ�
��2��A��B���ض�λ�ڳ��������A��Ϊ���ȶ�����ȴ���������B��ȴ���ȴ���ԭ��������Ҫԭ���Ǻ��θߣ�
��3����ͼ�п��Կ��������������ڷֲ��Ͼ��жԳ��ص㣮
��4�������ڷ����ȴ����ԭ�ϵĶ�����ʨ�ӡ�����������¹������ȣ�
��5�������������Ͼ��÷�չˮƽ��͵Ĵ��ޣ������ԭ���dz��ڵ�ֳ��ͳ�Σ�Ŀǰ������չ���õ�������������Դ�ḻ��
�ʴ�Ϊ��
��1��ʯ�ͣ����ʯ��ͭ��
��2�����θߣ�
��3���Գƣ�
��4��ʨ�ӡ�����������¹������
��4�����ڵ�ֳ��ͳ�Σ���Դ�ḻ��
�����������������½���в����ϱ��ع��ߴ��������ϱ����࣬���Է����ȴ��������Գ��Ϊ���ķֱ�Ϊ�ȴ����֡��ȴ���ԭ���ȴ�ɳĮ�����к������Ŀ����Դ����࣬����������ʯ���ƽ𡢸�����������εȵĴ����Ͳ�����������ǰ�У����ڳ��ڵ�ֳ��ͳ�Σ����ľ��÷�չ�ĺܻ�����������ᾭ�÷�չ������ʮ�ּ�ޣ�
���������⿼�������Դ��Ҫ�μǣ�

��ϰ��ϵ�д�
�����Ŀ
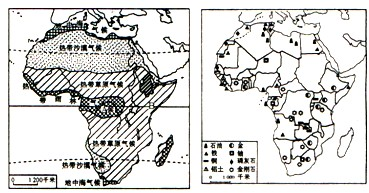 ����������ֲ�ͼ�Ϳ����Դ�ֲ�ͼ���ش��������⣺
����������ֲ�ͼ�Ϳ����Դ�ֲ�ͼ���ش��������⣺

