��Ŀ����
����Ŀ�����������ϵķ������������ް����ľ��ö����ڷ�չ�Σ������ġ���Ȼ��������Դ�������źܶ���֮ͬ�����ڷ�չ�п����������Ķ����в��ϣ��ش�����.
����һ��������������ͼ������½�س������ͼ������ͼ
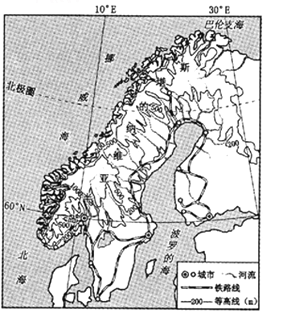
������������ͼ

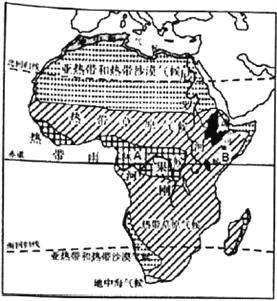
����½�س������ͼ ����ͼ
���϶�����Լ����¬����������
��1�����������ϵķ�����������__________���ֵĹ��磬�������������˿�������_______
___________________________________________��
��2�����������ϵķ��Ͱ���γ��λ�õĹ�ͬ����__________________________________��
��3��ͼ��B�ر�A���꽵ˮ���ٵ���ҪӰ��������___________________________________��
��4��ͼ�а������ڷֲ���ȫ��Լ60%���ȴ�����������Ϊ������֮�Ρ�����������Щ��Ļ���Ч�棿���������㣩_________________________________________________________
_________________________________________________________��
��5����ͼ˵������½��������ĵ����ص㣺_____________________________________��
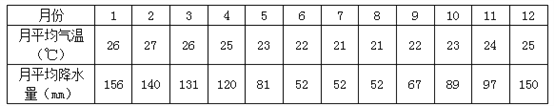
��6����31���ļ�����ƥ���˶��ᣬ��2016��8��5����21���ڰ�������Լ����¬���У����˻��ڼ䣬��Լ����¬�������ص���____________________________________________��
��7����ͼ���������������ͷֲ��ص�____________________________________________��ԭ��______________________________________________________________��
���𰸡���1����ɫ����Ѫ���������ࣻ ��2����λ���ȴ��� ��3����������
��4�����ϵ����ն�����̼��������в�������������������ˮԴ������ˮ���ȣ�
��5���������� ��6����������
(7)�Գ��Ϊ���ģ��ϱ��ԳƷֲ� ������������в����ϱ�����γ�ȴ������
�������� ���⿼�����������ϵķ���������ĵ����ſ���
��1�����������ϵķ����������Ϻ�ɫ���ֵĹ��磬�������������˿������ǻ�Ѫ���ֶࡣ
��2�����������ϵķ��Ͱ�������λ���ϱ��ع���֮�䣬���ֵ���λ���ȴ���
��3��ͼ��B��λ�ڰ��������Ǹ�ԭ�ϣ� A��λ�ڸչ���أ���B�꽵ˮ����A���ٵ���ҪӰ�������ǵ���������
��4��ͼ�а������ڷֲ���ȫ��Լ60%���ȴ����֣�����Ϊ������֮�Ρ�������Ч����������ϵ����ն�����̼��������в�������������������ˮԴ������ˮ���ȡ�
��5����ͼ��֪������½������������Զ�����������������ص㶫��������
��6����Լ����¬�����ȴ���ԭ���� 8��5�ա�21�����ϰ���Ķ��������ڰ�������Լ����¬���У����˻��ڼ䣬��Լ����¬�������ص��Ǹ���������
��7��������������в��������ϱ�����γ�ȴ�����ȣ��ʷ����������ͷֲ������Գ��Ϊ���ģ��ϱ��ԳƵ��ص㡣

 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�