��Ŀ����
���ҹ���һ�����¡��꽵ˮ���������ͷֲ�ͼ���ش��������⣮
��1�������������Ҫ�طֱ��� �� ��Ҫ��Ū����ҹ������ͱ���������������֣�
��2����ͼһ�е����ߵķֲ������Կ����ҹ�һ���ϱ����²��� ����Ҫԭ������ ���ص�Ӱ�죮
��3�����������ϱ������²����ũҵ����ʵ�ʣ��ҹ�����Ϊ����¶ȴ���ͼһ����ĸA������ ����
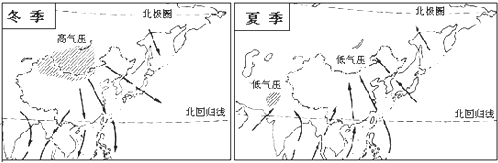
��4��ͼ�����ҹ���ˮ���ֲ�ͼ����ͼ�п��Կ����ҹ���ˮ�ķֲ������Ǵ� �� �ݼ����˱仯������Ҫ���� ���ص�Ӱ�죮
��5������ ���������Ķ��٣��ҹ�����Ϊ�ĸ���ʪ����������ɽ��ʡ���� ������
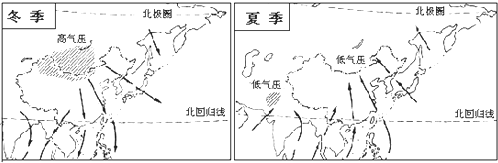
��6����γ��λ�úͺ�½λ�õ�Ӱ�죬�ҹ� ����������ͼ��B������������ ����Ӱ�����ǵ��ļ��������֣�ͼʾ����������̫ƽ��� �������磮�����ҹ��ļ���IJ��ȶ����ҹ����ļ�����������Ȼ�ֺ��� �ֺ���
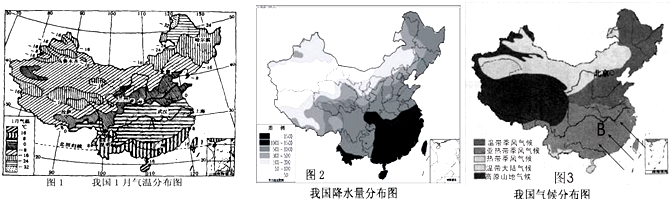
��1�������������Ҫ�طֱ���
��2����ͼһ�е����ߵķֲ������Կ����ҹ�һ���ϱ����²���
��3�����������ϱ������²����ũҵ����ʵ�ʣ��ҹ�����Ϊ����¶ȴ���ͼһ����ĸA������
��4��ͼ�����ҹ���ˮ���ֲ�ͼ����ͼ�п��Կ����ҹ���ˮ�ķֲ������Ǵ�
��5������
��6����γ��λ�úͺ�½λ�õ�Ӱ�죬�ҹ�

�����������뽵ˮ�����������Ӱ����̣���������Ϊ��ע������Ҫ�أ�
�����Ӷ��������ҹ��������Ҫ����֮һ��
�ҹ������ϱ����²�����Ϸ���ů����Խ�������¾�Խ�ͣ��ļ��ϱ��ձ���£�
�ҹ��������������������Ĺ���֮һ���ҹ����벿�д�Χ�ļ����������϶������ȴ������������ȴ����������´���������
�����Ӷ��������ҹ��������Ҫ����֮һ��
�ҹ������ϱ����²�����Ϸ���ů����Խ�������¾�Խ�ͣ��ļ��ϱ��ձ���£�
�ҹ��������������������Ĺ���֮һ���ҹ����벿�д�Χ�ļ����������϶������ȴ������������ȴ����������´���������
����⣺��1�������뽵ˮ�����������Ӱ����̣���������Ϊ��ע������Ҫ�أ�
��2��γ�����صIJ��죬��ֱ�ӵ����˽���̫������IJ�ͬ���϶�٣�ʹ�ҹ�һ���ϱ����²����
��3����ͼ��֪��ͼһ����ĸA���������ȴ���
��4����ͼ���п��Կ����ҹ���ˮ�ķֲ������ǴӶ��ϵ������ݼ����˱仯������Ҫ���ܺ�½���ص�Ӱ�죻
��5�����ݽ�ˮ�����������Ķ��٣��ҹ�����Ϊ�ĸ���ʪ����������ɽ��ʡ����ʪ��Ͱ�ʪ�������
��6���ҹ���������������ͼ��B���������������ȴ���������ͼʾ����������̫ƽ��Ķ��ϼ��磬�����ҹ��ļ���IJ��ȶ����ҹ����ļ������������ֺ���
�ʴ�Ϊ����1�����£���ˮ����2����γ��λ�ã���3�����ȣ���4�����ϣ���������½����5����ˮ����ʪ��Ͱ�ʪ��6�����磻���ȴ����磻���ϣ����ԣ�
��2��γ�����صIJ��죬��ֱ�ӵ����˽���̫������IJ�ͬ���϶�٣�ʹ�ҹ�һ���ϱ����²����
��3����ͼ��֪��ͼһ����ĸA���������ȴ���
��4����ͼ���п��Կ����ҹ���ˮ�ķֲ������ǴӶ��ϵ������ݼ����˱仯������Ҫ���ܺ�½���ص�Ӱ�죻
��5�����ݽ�ˮ�����������Ķ��٣��ҹ�����Ϊ�ĸ���ʪ����������ɽ��ʡ����ʪ��Ͱ�ʪ�������
��6���ҹ���������������ͼ��B���������������ȴ���������ͼʾ����������̫ƽ��Ķ��ϼ��磬�����ҹ��ļ���IJ��ȶ����ҹ����ļ������������ֺ���
�ʴ�Ϊ����1�����£���ˮ����2����γ��λ�ã���3�����ȣ���4�����ϣ���������½����5����ˮ����ʪ��Ͱ�ʪ��6�����磻���ȴ����磻���ϣ����ԣ�
���������⿼���ҹ������뽵ˮ���ص㣬Ҫ���������

��ϰ��ϵ�д�
�����Ŀ
�����ҹ��Ķ����ļ���ֲ�ʾ��ͼ�����±����ش�����
��1�������ҹ�λ��______��½��______��֮�䣬��ĺ�½��������ʹ�ֵ���һ���ڵķ����漾���������仯���γ��˵��͵ļ�������
��2�����ҹ��������ڣ�������������������½�ڲ���ƫ______�磬�Ӵ����ҹ����ֵ�������______�������½����ij̶ȣ��ҽ�ˮ______���ࡢ�٣����ļ������Ժ����ƫ______��Я���˷����ˮ�������ҹ��������γ��˴�����______�������ѩ����
��3�����ļ����Ӱ�죬�ҹ���ˮ��______�غ���______��½���٣��λ������ȫ�꽵ˮ������______����
| �ء����� | γ������ | һ��ƽ������ | ����ƽ������ |
| �������� | 39048��N | -4.70C | 260C |
| Ŧ����Լ | 40046��N | -0.50C | 24.60C |
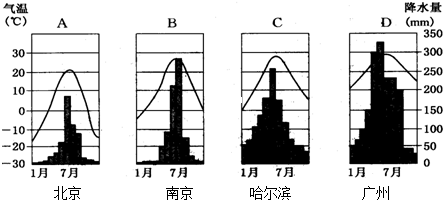
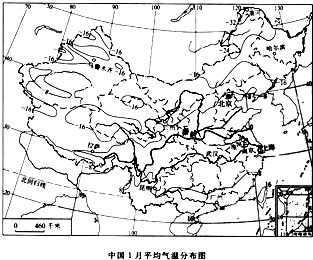 ���ҹ�һ�·�ƽ�����·ֲ�ͼ���ش����и��⣺
���ҹ�һ�·�ƽ�����·ֲ�ͼ���ش����и��⣺